 |
PDBsum entry 1m1m

|

|

|
|
 |
Contents |
 |
|
|

|

|
|
|
|
|
|
* Residue conservation analysis
|
|
|
|
 |
|
|
 |
 |
 |
 |
Enzyme class:
|
 |
E.C.2.3.1.301
- mycobacterial beta-ketoacyl-[acyl carrier protein] synthase Iii.
|
|
 |
 |
 |
 |
 |
Reaction:
|
 |
malonyl-[ACP] + dodecanoyl-CoA + H+ = 3-oxotetradecanoyl-[ACP] + CO2 + CoA
|
 |
 |
 |
 |
 |
malonyl-[ACP]
|
+
|
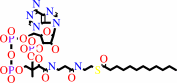 dodecanoyl-CoA
dodecanoyl-CoA
|
+
|
H(+)
|
=
|
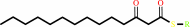 3-oxotetradecanoyl-[ACP]
3-oxotetradecanoyl-[ACP]
|
+
|
 CO2
CO2
|
+
|
 CoA
CoA
|
|
 |
 |
 |
 |
 |
 |
 |
 |
 |
 |
|
Molecule diagrams generated from .mol files obtained from the
KEGG ftp site
|
|
 |
 |
 |
 |
 |
 |
 |
 |
 |
 |
 |
 |
 |
 |
 |

|

|

|
| |
|
|
| |
|
DOI no:
|
J Biol Chem
280:32539-32547
(2005)
|
|
PubMed id:
|
|
|
|

|
| |
|
Probing the mechanism of the Mycobacterium tuberculosis beta-ketoacyl-acyl carrier protein synthase III mtFabH: factors influencing catalysis and substrate specificity.
|
|
A.K.Brown,
S.Sridharan,
L.Kremer,
S.Lindenberg,
L.G.Dover,
J.C.Sacchettini,
G.S.Besra.
|
|
|

|
| |
ABSTRACT
|
|

|
| |

|
Mycolic acids are the dominant feature of the Mycobacterium tuberculosis cell
wall. These alpha-alkyl, beta-hydroxy fatty acids are formed by the condensation
of two fatty acids, a long meromycolic acid and a shorter C(24)-C(26) fatty
acid. The component fatty acids are produced via a combination of type I and II
fatty acid synthases (FAS) with FAS-I products being elongated by FAS-II toward
meromycolic acids. The beta-ketoacyl-acyl carrier protein (ACP) synthase III
encoded by mtfabH (mtFabH) links FAS-I and FAS-II, catalyzing the condensation
of FAS-I-derived acyl-CoAs with malonyl-acyl carrier protein (ACP). The acyl-CoA
chain length specificity of mtFabH was assessed in vitro; the enzyme extended
longer, physiologically relevant acyl-CoA primers when paired with AcpM, its
natural partner, than with Escherichia coli ACP. The ability of the enzyme to
use E. coli ACP suggests that a similar mode of binding is likely with both
ACPs, yet it is clear that unique factors inherent to AcpM modulate the
substrate specificity of mtFabH. Mutation of proposed key mtFabH residues was
used to define their catalytic roles. Substitution of supposed acyl-CoA binding
residues reduced transacylation, with double substitutions totally abrogating
activity. Mutation of Arg(46) revealed its more critical role in malonyl-AcpM
decarboxylation than in the acyl-CoA binding role. Interestingly, this effect
was suppressed intragenically by Arg(161) --> Ala substitution. Our structural
studies suggested that His(258), previously implicated in malonyl-ACP
decarboxylation, also acts as an anchor point for a network of water molecules
that we propose promotes deprotonation and transacylation of Cys(122).

|

|
|
|

|
| |
Selected figure(s)
|
|

|
| |
 |
 |

|
 |

|
 |
Figure 5.
FIGURE 5. Conserved water molecules near the active site
residues in mtFabH structures. Arg46-Arg161  Ala mutant (blue) and
1HZP (magenta) structures are superposed. Residues and water
(wat) molecules are numbered as in the Arg46-Arg161 Ala mutant (blue) and
1HZP (magenta) structures are superposed. Residues and water
(wat) molecules are numbered as in the Arg46-Arg161  Ala
mutant structure. The figure was made using Xtalview (37). Ala
mutant structure. The figure was made using Xtalview (37).
|
 |
Figure 6.
FIGURE 6. Proposed mechanism for mtFabH transacylation. The
formation of the thiolate ion at Cys122, which is crucial to the
transacylation reaction, appears to be promoted in part by the
helix dipole effect (represented here by a partial positive
charge at the N-terminal end of helix 5) and by shuttling of the
proton via water 568 and ultimately abstraction via N  2 of
His258. Through hydrogen bonding, the backbone nitrogens of
Gly322 and Cys122 stabilize the negative charge gained by the
acyl-CoA carbonyl during formation of the acyl-enzyme thioester
Michaelis complex (boxed). Data are adapted from Refs. 24 and 29. 2 of
His258. Through hydrogen bonding, the backbone nitrogens of
Gly322 and Cys122 stabilize the negative charge gained by the
acyl-CoA carbonyl during formation of the acyl-enzyme thioester
Michaelis complex (boxed). Data are adapted from Refs. 24 and 29.
|
 |
|
|

|
| |
The above figures are
reprinted
by permission from the ASBMB:
J Biol Chem
(2005,
280,
32539-32547)
copyright 2005.
|
|
| |
Figures were
selected
by an automated process.
|
|

|

|
|
 |
 |
|
 |
 |
 |
 |
 |
 |
 |
 |
 |
|
Literature references that cite this PDB file's key reference
|
|
 |
| |
PubMed id
|
 |
Reference
|
 |
|
|
|
 |
V.Molle,
G.Gulten,
C.Vilchèze,
R.Veyron-Churlet,
I.Zanella-Cléon,
J.C.Sacchettini,
W.R.Jacobs,
and
L.Kremer
(2010).
Phosphorylation of InhA inhibits mycolic acid biosynthesis and growth of Mycobacterium tuberculosis.
|
| |
Mol Microbiol,
78,
1591-1605.
|
 |
|
PDB codes:
|
 |
|
|
|
|
|
 |
A.K.Brown,
R.C.Taylor,
A.Bhatt,
K.Fütterer,
and
G.S.Besra
(2009).
Platensimycin activity against mycobacterial beta-ketoacyl-ACP synthases.
|
| |
PLoS One,
4,
e6306.
|
 |
|
|
|
|
 |
Q.Al-Balas,
N.G.Anthony,
B.Al-Jaidi,
A.Alnimr,
G.Abbott,
A.K.Brown,
R.C.Taylor,
G.S.Besra,
T.D.McHugh,
S.H.Gillespie,
B.F.Johnston,
S.P.Mackay,
and
G.D.Coxon
(2009).
Identification of 2-Aminothiazole-4-Carboxylate Derivatives Active against Mycobacterium tuberculosis H(37)R(v) and the beta-Ketoacyl-ACP Synthase mtFabH.
|
| |
PLoS ONE,
4,
e5617.
|
 |
|
|
|
|
 |
R.Veyron-Churlet,
V.Molle,
R.C.Taylor,
A.K.Brown,
G.S.Besra,
I.Zanella-Cléon,
K.Fütterer,
and
L.Kremer
(2009).
The Mycobacterium tuberculosis beta-ketoacyl-acyl carrier protein synthase III activity is inhibited by phosphorylation on a single threonine residue.
|
| |
J Biol Chem,
284,
6414-6424.
|
 |
|
|
|
|
 |
A.Gurvitz,
J.K.Hiltunen,
and
A.J.Kastaniotis
(2008).
Function of heterologous Mycobacterium tuberculosis InhA, a type 2 fatty acid synthase enzyme involved in extending C20 fatty acids to C60-to-C90 mycolic acids, during de novo lipoic acid synthesis in Saccharomyces cerevisiae.
|
| |
Appl Environ Microbiol,
74,
5078-5085.
|
 |
|
|
|
|
 |
R.Goude,
and
T.Parish
(2008).
The genetics of cell wall biosynthesis in Mycobacterium tuberculosis.
|
| |
Future Microbiol,
3,
299-313.
|
 |
|
|
|
|
 |
S.Sachdeva,
F.Musayev,
M.M.Alhamadsheh,
J.Neel Scarsdale,
H.Tonie Wright,
and
K.A.Reynolds
(2008).
Probing reactivity and substrate specificity of both subunits of the dimeric Mycobacterium tuberculosis FabH using alkyl-CoA disulfide inhibitors and acyl-CoA substrates.
|
| |
Bioorg Chem,
36,
85-90.
|
 |
|
PDB code:
|
 |
|
|
|
|
|
 |
V.Bhowruth,
A.K.Brown,
and
G.S.Besra
(2008).
Synthesis and biological evaluation of NAS-21 and NAS-91 analogues as potential inhibitors of the mycobacterial FAS-II dehydratase enzyme Rv0636.
|
| |
Microbiology,
154,
1866-1875.
|
 |
|
|
|
|
 |
A.Bhatt,
V.Molle,
G.S.Besra,
W.R.Jacobs,
and
L.Kremer
(2007).
The Mycobacterium tuberculosis FAS-II condensing enzymes: their role in mycolic acid biosynthesis, acid-fastness, pathogenesis and in future drug development.
|
| |
Mol Microbiol,
64,
1442-1454.
|
 |
|
|
|
|
 |
H.Ghadbane,
A.K.Brown,
L.Kremer,
G.S.Besra,
and
K.Fütterer
(2007).
Structure of Mycobacterium tuberculosis mtFabD, a malonyl-CoA:acyl carrier protein transacylase (MCAT).
|
| |
Acta Crystallogr Sect F Struct Biol Cryst Commun,
63,
831-835.
|
 |
|
PDB code:
|
 |
|
|
|
|
|
 |
M.M.Alhamadsheh,
F.Musayev,
A.A.Komissarov,
S.Sachdeva,
H.T.Wright,
N.Scarsdale,
G.Florova,
and
K.A.Reynolds
(2007).
Alkyl-CoA disulfides as inhibitors and mechanistic probes for FabH enzymes.
|
| |
Chem Biol,
14,
513-524.
|
 |
|
PDB codes:
|
 |
|
|
|
|
|
 |
S.Sridharan,
L.Wang,
A.K.Brown,
L.G.Dover,
L.Kremer,
G.S.Besra,
and
J.C.Sacchettini
(2007).
X-ray crystal structure of Mycobacterium tuberculosis beta-ketoacyl acyl carrier protein synthase II (mtKasB).
|
| |
J Mol Biol,
366,
469-480.
|
 |
|
PDB code:
|
 |
|
|
 |
 |
|
The most recent references are shown first.
Citation data come partly from CiteXplore and partly
from an automated harvesting procedure. Note that this is likely to be
only a partial list as not all journals are covered by
either method. However, we are continually building up the citation data
so more and more references will be included with time.
Where a reference describes a PDB structure, the PDB
codes are
shown on the right.
|
 |

