 |
PDBsum entry 1m1b

|

|

|
|
 |
Contents |
 |
|
|

|

|
|
|
|
|
|
|
|
|
|
* Residue conservation analysis
|
|
|
|
 |
|
|
 |
 |
 |
 |
Enzyme class:
|
 |
E.C.5.4.2.9
- phosphoenolpyruvate mutase.
|
|
 |
 |
 |
 |
 |

Pathway:
|
 |
Phosphoenolpyruvate Mutase
|
 |
 |
 |
 |
 |
Reaction:
|
 |
phosphoenolpyruvate + H+ = 3-phosphonopyruvate
|
 |
 |
 |
 |
 |
 phosphoenolpyruvate
phosphoenolpyruvate
|
+
|
H(+)
Bound ligand (Het Group name = )
matches with 42.86% similarity
|
=
|
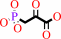 3-phosphonopyruvate
3-phosphonopyruvate
|
|
 |
 |
 |
 |
 |
 |
 |
 |
 |
 |
|
Molecule diagrams generated from .mol files obtained from the
KEGG ftp site
|
|
 |
 |
 |
 |
 |
 |
 |
 |
 |
 |
 |
 |
 |
 |
 |

|

|

|
| |
|
|
| |
|
DOI no:
|
Biochemistry
41:10270-10276
(2002)
|
|
PubMed id:
|
|
|
|

|
| |
|
Dissociative phosphoryl transfer in PEP mutase catalysis: structure of the enzyme/sulfopyruvate complex and kinetic properties of mutants.
|
|
S.Liu,
Z.Lu,
Y.Jia,
D.Dunaway-Mariano,
O.Herzberg.
|
|
|

|
| |
ABSTRACT
|
|

|
| |

|
The crystal structure of PEP mutase from Mytilus edulis in complex with a
substrate-analogue inhibitor, sulfopyruvate S-pyr (K(i) = 22 microM), has been
determined at 2.25 A resolution. Mg(II)-S-pyr binds in the alpha/beta barrel's
central channel, at the C-termini of the beta-strands. The binding mode of
S-pyr's pyruvyl moiety resembles the binding mode of oxalate seen earlier. The
location of the sulfo group of S-pyr is postulated to mimic the phosphonyl group
of the product phosphonopyruvate (P-pyr). This sulfo group interacts with the
guanidinium group of Arg159, but it is not aligned for nucleopilic attack by
neighboring basic amino side chains. Kinetic analysis of site directed mutants,
probing the key active site residues Asp58, Arg159, Asn122, and His190 correlate
well with the structural information. The results presented here rule out a
phosphoryl transfer mechanism involving a double displacement, and suggest
instead that PEP mutase catalysis proceeds via a dissociative mechanism in which
the pyruvyl C(3) adds to the same face of the phosphorus from which the C(2)O
departs. We propose that Arg159 and His190 serve to hold the
phosphoryl/metaphosphate/phosphonyl group stationary along the reaction pathway,
while the pyruvyl C(1)-C(2) bond rotates upon formation of the metaphosphate. In
agreement with published data, the phosphoryl group transfer occurs on the
Si-face of PEP with retention of configuration at phosphorus.

|

|
|
|

|

|
|
 |
 |
|
 |
 |
 |
 |
 |
 |
 |
 |
 |
|
Literature references that cite this PDB file's key reference
|
|
 |
| |
PubMed id
|
 |
Reference
|
 |
|
|
|
 |
W.W.Metcalf,
and
W.A.van der Donk
(2009).
Biosynthesis of phosphonic and phosphinic acid natural products.
|
| |
Annu Rev Biochem,
78,
65-94.
|
 |
|
|
|
|
 |
B.C.Narayanan,
W.Niu,
Y.Han,
J.Zou,
P.S.Mariano,
D.Dunaway-Mariano,
and
O.Herzberg
(2008).
Structure and function of PA4872 from Pseudomonas aeruginosa, a novel class of oxaloacetate decarboxylase from the PEP mutase/isocitrate lyase superfamily.
|
| |
Biochemistry,
47,
167-182.
|
 |
|
PDB code:
|
 |
|
|
|
|
|
 |
C.J.Liao,
K.H.Chin,
C.H.Lin,
P.S.Tsai,
P.C.Lyu,
C.C.Young,
A.H.Wang,
and
S.H.Chou
(2008).
Crystal structure of DFA0005 complexed with alpha-ketoglutarate: a novel member of the ICL/PEPM superfamily from alkali-tolerant Deinococcus ficus.
|
| |
Proteins,
73,
362-371.
|
 |
|
PDB code:
|
 |
|
|
|
|
|
 |
J.W.Munos,
S.J.Moon,
S.O.Mansoorabadi,
W.Chang,
L.Hong,
F.Yan,
A.Liu,
and
H.W.Liu
(2008).
Purification and characterization of the epoxidase catalyzing the formation of fosfomycin from Pseudomonas syringae.
|
| |
Biochemistry,
47,
8726-8735.
|
 |
|
|
|
|
 |
I.Ntai,
V.V.Phelan,
and
B.O.Bachmann
(2006).
Phosphonopeptide K-26 biosynthetic intermediates in Astrosporangium hypotensionis.
|
| |
Chem Commun (Camb),
(),
4518-4520.
|
 |
|
|
|
|
 |
J.G.Zalatan,
and
D.Herschlag
(2006).
Alkaline phosphatase mono- and diesterase reactions: comparative transition state analysis.
|
| |
J Am Chem Soc,
128,
1293-1303.
|
 |
|
|
|
|
 |
G.Zhang,
J.Dai,
Z.Lu,
and
D.Dunaway-Mariano
(2003).
The phosphonopyruvate decarboxylase from Bacteroides fragilis.
|
| |
J Biol Chem,
278,
41302-41308.
|
 |
|
|
|
|
 |
P.Liu,
A.Liu,
F.Yan,
M.D.Wolfe,
J.D.Lipscomb,
and
H.W.Liu
(2003).
Biochemical and spectroscopic studies on (S)-2-hydroxypropylphosphonic acid epoxidase: a novel mononuclear non-heme iron enzyme.
|
| |
Biochemistry,
42,
11577-11586.
|
 |
|
|
|
|
 |
T.L.Grimek,
H.Holden,
I.Rayment,
and
J.C.Escalante-Semerena
(2003).
Residues C123 and D58 of the 2-methylisocitrate lyase (PrpB) enzyme of Salmonella enterica are essential for catalysis.
|
| |
J Bacteriol,
185,
4837-4843.
|
 |
|
 |
 |
|
The most recent references are shown first.
Citation data come partly from CiteXplore and partly
from an automated harvesting procedure. Note that this is likely to be
only a partial list as not all journals are covered by
either method. However, we are continually building up the citation data
so more and more references will be included with time.
Where a reference describes a PDB structure, the PDB
code is
shown on the right.
|
 |

