 |
PDBsum entry 1lc8

|

|

|
|
 |
Contents |
 |
|
|

|

|
|
|
|
|
|
|
|
* Residue conservation analysis
|
|
|
|
 |
|
|
 |
 |
 |
 |
Enzyme class:
|
 |
E.C.4.1.1.81
- threonine-phosphate decarboxylase.
|
|
 |
 |
 |
 |
 |

Pathway:
|
 |
Corrin Biosynthesis (part 6)
|
 |
 |
 |
 |
 |
Reaction:
|
 |
O-phospho-L-threonine + H+ = (R)-1-aminopropan-2-yl phosphate + CO2
|
 |
 |
 |
 |
 |
O-phospho-L-threonine
|
+
|
H(+)
|
=
|
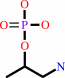 (R)-1-aminopropan-2-yl phosphate
(R)-1-aminopropan-2-yl phosphate
|
+
|
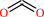 CO2
CO2
|
|
 |
 |
 |
 |
 |
 |
 |
 |
Cofactor:
|
 |
Pyridoxal 5'-phosphate
|
 |
 |
 |
 |
 |
Pyridoxal 5'-phosphate
Bound ligand (Het Group name =
33P)
matches with 60.00% similarity
|
|
 |
 |
 |
 |
|
Molecule diagrams generated from .mol files obtained from the
KEGG ftp site
|
|
 |
 |
 |
 |
 |
 |
 |
 |
 |
 |
 |
 |
 |
 |
 |

|

|

|
| |
|
|
| |
|
DOI no:
|
Biochemistry
41:9079-9089
(2002)
|
|
PubMed id:
|
|
|
|

|
| |
|
Structural studies of the L-threonine-O-3-phosphate decarboxylase (CobD) enzyme from Salmonella enterica: the apo, substrate, and product-aldimine complexes.
|
|
C.G.Cheong,
J.C.Escalante-Semerena,
I.Rayment.
|
|
|

|
| |
ABSTRACT
|
|

|
| |

|
The evolution of biosynthetic pathways is difficult to reconstruct in hindsight;
however, the structures of the enzymes that are involved may provide insight
into their development. One enzyme in the cobalamin biosynthetic pathway that
appears to have evolved from a protein with different function is
L-threonine-O-3-phosphate decarboxylase (CobD) from Salmonella enterica, which
is structurally similar to histidinol phosphate aminotransferase [Cheong, C. G.,
Bauer, C. B., Brushaber, K. R., Escalante-Semerena, J. C., and Rayment, I.
(2002) Biochemistry 41, 4798-4808]. This enzyme is responsible for synthesizing
(R)-1-amino-2-propanol phosphate which is the precursor for the linkage between
the nucleotide loop and the corrin ring in cobalamin. To understand the
relationship between this decarboxylase and the aspartate aminotransferase
family to which it belongs, the structures of CobD in its apo state, the apo
state complexed with the substrate, and its product external aldimine complex
have been determined at 1.46, 1.8, and 1.8 A resolution, respectively. These
structures show that the enzyme steers the breakdown of the external aldimine
toward decarboxylation instead of amino transfer by positioning the carboxylate
moiety of the substrate out of the plane of the pyridoxal ring and by placing
the alpha-hydrogen out of reach of the catalytic base provided by the lysine
that forms the internal aldimine. It would appear that CobD evolved from a
primordial PLP-dependent aminotransferase, where the selection was based on
similarities between the stereochemical properties of the substrates rather than
preservation of the fate of the external aldimine. These structures provide a
sequence signature for distinguishing between L-threonine-O-3-phosphate
decarboxylase and histidinol phosphate aminotransferases, many of which appear
to have been misannotated.

|

|
|
|

|

|
|
 |
 |
|
 |
 |
 |
 |
 |
 |
 |
 |
 |
|
Literature references that cite this PDB file's key reference
|
|
 |
| |
PubMed id
|
 |
Reference
|
 |
|
|
|
 |
J.Marienhagen,
T.Sandalova,
H.Sahm,
L.Eggeling,
and
G.Schneider
(2008).
Insights into the structural basis of substrate recognition by histidinol-phosphate aminotransferase from Corynebacterium glutamicum.
|
| |
Acta Crystallogr D Biol Crystallogr,
64,
675-685.
|
 |
|
PDB codes:
|
 |
|
|
 |
 |
|
The most recent references are shown first.
Citation data come partly from CiteXplore and partly
from an automated harvesting procedure. Note that this is likely to be
only a partial list as not all journals are covered by
either method. However, we are continually building up the citation data
so more and more references will be included with time.
Where a reference describes a PDB structure, the PDB
codes are
shown on the right.
|
 |

