 |
PDBsum entry 1l7o

|

|

|
|
 |
Contents |
 |
|
|

|

|
|
|
|
|
|
|
|
|
|
* Residue conservation analysis
|
|
|

|
|
PDB id:
|
 |
|
 |
| Name: |
 |
Hydrolase
|
 |
|
Title:
|
 |
Crystal structure of phosphoserine phosphatase in apo form
|
|
Structure:
|
 |
Phosphoserine phosphatase. Chain: a, b. Synonym: psp, o-phosphoserine phosphohydrolase, pspase. Engineered: yes. Mutation: yes
|
|
Source:
|
 |
Methanocaldococcus jannaschii. Organism_taxid: 2190. Gene: mj1594. Expressed in: escherichia coli. Expression_system_taxid: 562.
|
|
Resolution:
|
 |
|
2.20Å
|
R-factor:
|
0.224
|
R-free:
|
0.254
|
|
|
Authors:
|
 |
W.Wang,H.S.Cho,R.Kim,J.Jancarik,H.Yokota,H.H.Nguyen,I.V.Grigoriev, D.E.Wemmer,S.H.Kim,Berkeley Structural Genomics Center (Bsgc)
|
Key ref:

|
 |
W.Wang
et al.
(2002).
Structural characterization of the reaction pathway in phosphoserine phosphatase: crystallographic "snapshots" of intermediate states.
J Mol Biol,
319,
421-431.
PubMed id:
DOI:

|
 |
|
Date:
|
 |
|
16-Mar-02
|
Release date:
|
19-Jun-02
|
|
|

|

|
|
PROCHECK
|

|

|
|
|
Headers
|
 |
|
|
References
|
|
|
|

|

|
|
Q58989
(SERB_METJA) -
Phosphoserine phosphatase from Methanocaldococcus jannaschii (strain ATCC 43067 / DSM 2661 / JAL-1 / JCM 10045 / NBRC 100440)
|
|

|

|
Seq:
Struc:
|
 |
 |
 |
211 a.a.
200 a.a.*
|

|

|

|

|
|

|

|

|
 |
 |

|
|
Key: |
 |
PfamA domain |
 |
 |
 |
Secondary structure |
 |
 |
CATH domain |
 |
|
*
PDB and UniProt seqs differ
at 1 residue position (black
cross)
|
|

|
|
|
 |
|
|
 |
 |
 |
 |
Enzyme class:
|
 |
E.C.3.1.3.3
- phosphoserine phosphatase.
|
|
 |
 |
 |
 |
 |
Reaction:
|
 |
|
1.
|
O-phospho-L-serine + H2O = L-serine + phosphate
|
|
2.
|
O-phospho-D-serine + H2O = D-serine + phosphate
|
|
 |
 |
 |
 |
 |
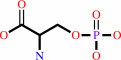 O-phospho-L-serine
O-phospho-L-serine
|
+
|
H2O
|
=
|
L-serine
Bound ligand (Het Group name = )
matches with 57.14% similarity
|
+
|
 phosphate
phosphate
|
|
 |
 |
 |
 |
 |
O-phospho-D-serine
|
+
|
H2O
|
=
|
D-serine
Bound ligand (Het Group name = )
matches with 57.14% similarity
|
+
|
 phosphate
phosphate
|
|
 |
 |
 |
 |
 |
 |
 |
 |
 |
 |
|
Molecule diagrams generated from .mol files obtained from the
KEGG ftp site
|
|
 |
 |
 |
 |
 |
 |
 |
 |
 |
 |
 |
 |
 |
 |
 |

|

|

|
| |
|
|
| |
|
DOI no:
|
J Mol Biol
319:421-431
(2002)
|
|
PubMed id:
|
|
|
|

|
| |
|
Structural characterization of the reaction pathway in phosphoserine phosphatase: crystallographic "snapshots" of intermediate states.
|
|
W.Wang,
H.S.Cho,
R.Kim,
J.Jancarik,
H.Yokota,
H.H.Nguyen,
I.V.Grigoriev,
D.E.Wemmer,
S.H.Kim.
|
|
|

|
| |
ABSTRACT
|
|

|
| |

|
Phosphoserine phosphatase (PSP) is a member of a large class of enzymes that
catalyze phosphoester hydrolysis using a phosphoaspartate-enzyme intermediate.
PSP is a likely regulator of the steady-state d-serine level in the brain, which
is a critical co-agonist of the N-methyl-d-aspartate type of glutamate
receptors. Here, we present high-resolution (1.5-1.9 A) structures of PSP from
Methanococcus jannaschii, which define the open state prior to substrate
binding, the complex with phosphoserine substrate bound (with a D to N mutation
in the active site), and the complex with AlF3, a transition-state analog for
the phospho-transfer steps in the reaction. These structures, together with
those described for the BeF3- complex (mimicking the phospho-enzyme) and the
enzyme with phosphate product in the active site, provide a detailed structural
picture of the full reaction cycle. The structure of the apo state indicates
partial unfolding of the enzyme to allow substrate binding, with refolding in
the presence of substrate to provide specificity. Interdomain and active-site
conformational changes are identified. The structure with the transition state
analog bound indicates a "tight" intermediate. A striking structure
homology, with significant sequence conservation, among PSP, P-type ATPases and
response regulators suggests that the knowledge of the PSP reaction mechanism
from the structures determined will provide insights into the reaction
mechanisms of the other enzymes in this family.

|

|
|
|

|
| |
Selected figure(s)
|
|

|
| |
 |
 |

|
 |

|
 |
Figure 3.
Figure 3. PSP structure: (a) a ribbon diagram; (b) a
topology diagram.
|
 |
Figure 5.
Figure 5. Structural "snapshots" of the PSP reaction cycle
in the active site. (a) The PSP reaction cycle. (b) Structure
and models in the active site. (I) The apo-enzyme structure.
(II) The substrate, PLS, bound structure, using mutant D11N.
(III) The model of Ser bound transition state structural
analogue. A Ser molecule was modeled in the AlF[3]-PSP active
site. (IV) Phospho-aspartyl enzyme intermediate structural
analogue. The PSP+BeF[3]^ - complex. (V) The transition state
structural analogue. AlF[3]-PSP complex. (VI) The product, Pi,
bound structure.
|
 |
|
|

|
| |
The above figures are
reprinted
by permission from Elsevier:
J Mol Biol
(2002,
319,
421-431)
copyright 2002.
|
|
| |
Figures were
selected
by the author.
|
|

|

|
|
 |
 |
|
 |
 |
 |
 |
 |
 |
 |
 |
 |
|
Literature references that cite this PDB file's key reference
|
|
 |
| |
PubMed id
|
 |
Reference
|
 |
|
|
|
 |
P.Wei,
L.C.Milbauer,
J.Enenstein,
J.Nguyen,
W.Pan,
and
R.P.Hebbel
(2011).
Differential endothelial cell gene expression by African Americans versus Caucasian Americans: a possible contribution to health disparity in vascular disease and cancer.
|
| |
BMC Med,
9,
2.
|
 |
|
|
|
|
 |
S.Re,
T.Imai,
J.Jung,
S.Ten-No,
and
Y.Sugita
(2011).
Geometrically associative yet electronically dissociative character in the transition state of enzymatic reversible phosphorylation.
|
| |
J Comput Chem,
32,
260-270.
|
 |
|
|
|
|
 |
H.H.Nguyen,
L.Wang,
H.Huang,
E.Peisach,
D.Dunaway-Mariano,
and
K.N.Allen
(2010).
Structural determinants of substrate recognition in the HAD superfamily member D-glycero-D-manno-heptose-1,7-bisphosphate phosphatase (GmhB) .
|
| |
Biochemistry,
49,
1082-1092.
|
 |
|
PDB codes:
|
 |
|
|
|
|
|
 |
H.Suzuki,
K.Yamasaki,
T.Daiho,
and
S.Danko
(2010).
[Mechanism of ca(2+) pump as revealed by mutations, development of stable analogs of phosphorylated intermediates, and their structural analyses].
|
| |
Yakugaku Zasshi,
130,
179-189.
|
 |
|
|
|
|
 |
J.V.Møller,
C.Olesen,
A.M.Winther,
and
P.Nissen
(2010).
The sarcoplasmic Ca2+-ATPase: design of a perfect chemi-osmotic pump.
|
| |
Q Rev Biophys,
43,
501-566.
|
 |
|
|
|
|
 |
R.B.Bourret
(2010).
Receiver domain structure and function in response regulator proteins.
|
| |
Curr Opin Microbiol,
13,
142-149.
|
 |
|
|
|
|
 |
Y.Pazy,
M.A.Motaleb,
M.T.Guarnieri,
N.W.Charon,
R.Zhao,
and
R.E.Silversmith
(2010).
Identical phosphatase mechanisms achieved through distinct modes of binding phosphoprotein substrate.
|
| |
Proc Natl Acad Sci U S A,
107,
1924-1929.
|
 |
|
PDB code:
|
 |
|
|
|
|
|
 |
J.Dai,
L.Finci,
C.Zhang,
S.Lahiri,
G.Zhang,
E.Peisach,
K.N.Allen,
and
D.Dunaway-Mariano
(2009).
Analysis of the structural determinants underlying discrimination between substrate and solvent in beta-phosphoglucomutase catalysis.
|
| |
Biochemistry,
48,
1984-1995.
|
 |
|
PDB code:
|
 |
|
|
|
|
|
 |
K.O.Håkansson
(2009).
The structure of Mg-ATPase nucleotide-binding domain at 1.6 A resolution reveals a unique ATP-binding motif.
|
| |
Acta Crystallogr D Biol Crystallogr,
65,
1181-1186.
|
 |
|
PDB code:
|
 |
|
|
|
|
|
 |
M.Decker,
M.Arand,
and
A.Cronin
(2009).
Mammalian epoxide hydrolases in xenobiotic metabolism and signalling.
|
| |
Arch Toxicol,
83,
297-318.
|
 |
|
|
|
|
 |
S.Danko,
T.Daiho,
K.Yamasaki,
X.Liu,
and
H.Suzuki
(2009).
Formation of the stable structural analog of ADP-sensitive phosphoenzyme of Ca2+-ATPase with occluded Ca2+ by beryllium fluoride: structural changes during phosphorylation and isomerization.
|
| |
J Biol Chem,
284,
22722-22735.
|
 |
|
|
|
|
 |
S.Michielssens,
N.Tien Trung,
M.Froeyen,
P.Herdewijn,
M.Tho Nguyen,
and
A.Ceulemans
(2009).
Hydrolysis of aspartic acid phosphoramidate nucleotides: a comparative quantum chemical study.
|
| |
Phys Chem Chem Phys,
11,
7274-7285.
|
 |
|
|
|
|
 |
Y.Pazy,
A.C.Wollish,
S.A.Thomas,
P.J.Miller,
E.J.Collins,
R.B.Bourret,
and
R.E.Silversmith
(2009).
Matching biochemical reaction kinetics to the timescales of life: structural determinants that influence the autodephosphorylation rate of response regulator proteins.
|
| |
J Mol Biol,
392,
1205-1220.
|
 |
|
PDB codes:
|
 |
|
|
|
|
|
 |
A.Ghosh,
S.Shuman,
and
C.D.Lima
(2008).
The structure of Fcp1, an essential RNA polymerase II CTD phosphatase.
|
| |
Mol Cell,
32,
478-490.
|
 |
|
PDB codes:
|
 |
|
|
|
|
|
 |
S.A.Thomas,
J.A.Brewster,
and
R.B.Bourret
(2008).
Two variable active site residues modulate response regulator phosphoryl group stability.
|
| |
Mol Microbiol,
69,
453-465.
|
 |
|
|
|
|
 |
T.Kawamura,
N.Watanabe,
and
I.Tanaka
(2008).
Structure of mannosyl-3-phosphoglycerate phosphatase from Pyrococcus horikoshii.
|
| |
Acta Crystallogr D Biol Crystallogr,
64,
1267-1276.
|
 |
|
PDB codes:
|
 |
|
|
|
|
|
 |
Z.Lu,
D.Dunaway-Mariano,
and
K.N.Allen
(2008).
The catalytic scaffold of the haloalkanoic acid dehalogenase enzyme superfamily acts as a mold for the trigonal bipyramidal transition state.
|
| |
Proc Natl Acad Sci U S A,
105,
5687-5692.
|
 |
|
PDB codes:
|
 |
|
|
|
|
|
 |
A.K.Hirsch,
F.R.Fischer,
and
F.Diederich
(2007).
Phosphate recognition in structural biology.
|
| |
Angew Chem Int Ed Engl,
46,
338-352.
|
 |
|
|
|
|
 |
C.Toyoshima,
Y.Norimatsu,
S.Iwasawa,
T.Tsuda,
and
H.Ogawa
(2007).
How processing of aspartylphosphate is coupled to lumenal gating of the ion pathway in the calcium pump.
|
| |
Proc Natl Acad Sci U S A,
104,
19831-19836.
|
 |
|
PDB codes:
|
 |
|
|
|
|
|
 |
Q.Xu,
K.S.Saikatendu,
S.S.Krishna,
D.McMullan,
P.Abdubek,
S.Agarwalla,
E.Ambing,
T.Astakhova,
H.L.Axelrod,
D.Carlton,
H.J.Chiu,
T.Clayton,
M.DiDonato,
L.Duan,
M.A.Elsliger,
J.Feuerhelm,
S.K.Grzechnik,
J.Hale,
E.Hampton,
G.W.Han,
J.Haugen,
L.Jaroszewski,
K.K.Jin,
H.E.Klock,
M.W.Knuth,
E.Koesema,
M.D.Miller,
A.T.Morse,
E.Nigoghossian,
L.Okach,
S.Oommachen,
J.Paulsen,
R.Reyes,
C.L.Rife,
R.Schwarzenbacher,
H.van den Bedem,
A.White,
G.Wolf,
K.O.Hodgson,
J.Wooley,
A.M.Deacon,
A.Godzik,
S.A.Lesley,
and
I.A.Wilson
(2007).
Crystal structure of MtnX phosphatase from Bacillus subtilis at 2.0 A resolution provides a structural basis for bipartite phosphomonoester hydrolysis of 2-hydroxy-3-keto-5-methylthiopentenyl-1-phosphate.
|
| |
Proteins,
69,
433-439.
|
 |
|
PDB code:
|
 |
|
|
|
|
|
 |
S.C.Kamerlin,
and
J.Wilkie
(2007).
The role of metal ions in phosphate ester hydrolysis.
|
| |
Org Biomol Chem,
5,
2098-2108.
|
 |
|
|
|
|
 |
S.Helgadóttir,
G.Rosas-Sandoval,
D.Söll,
and
D.E.Graham
(2007).
Biosynthesis of phosphoserine in the Methanococcales.
|
| |
J Bacteriol,
189,
575-582.
|
 |
|
|
|
|
 |
Y.Hatori,
E.Majima,
T.Tsuda,
and
C.Toyoshima
(2007).
Domain organization and movements in heavy metal ion pumps: papain digestion of CopA, a Cu+-transporting ATPase.
|
| |
J Biol Chem,
282,
25213-25221.
|
 |
|
|
|
|
 |
E.Bitto,
C.A.Bingman,
G.E.Wesenberg,
J.G.McCoy,
and
G.N.Phillips
(2006).
Structure of pyrimidine 5'-nucleotidase type 1. Insight into mechanism of action and inhibition during lead poisoning.
|
| |
J Biol Chem,
281,
20521-20529.
|
 |
|
PDB codes:
|
 |
|
|
|
|
|
 |
E.S.Groban,
A.Narayanan,
and
M.P.Jacobson
(2006).
Conformational changes in protein loops and helices induced by post-translational phosphorylation.
|
| |
PLoS Comput Biol,
2,
e32.
|
 |
|
|
|
|
 |
E.S.Rangarajan,
A.Proteau,
J.Wagner,
M.N.Hung,
A.Matte,
and
M.Cygler
(2006).
Structural snapshots of Escherichia coli histidinol phosphate phosphatase along the reaction pathway.
|
| |
J Biol Chem,
281,
37930-37941.
|
 |
|
PDB codes:
|
 |
|
|
|
|
|
 |
J.D.Clausen,
D.B.McIntosh,
D.G.Woolley,
A.N.Anthonisen,
B.Vilsen,
and
J.P.Andersen
(2006).
Asparagine 706 and glutamate 183 at the catalytic site of sarcoplasmic reticulum Ca2+-ATPase play critical but distinct roles in E2 states.
|
| |
J Biol Chem,
281,
9471-9481.
|
 |
|
|
|
|
 |
K.N.Rao,
D.Kumaran,
J.Seetharaman,
J.B.Bonanno,
S.K.Burley,
and
S.Swaminathan
(2006).
Crystal structure of trehalose-6-phosphate phosphatase-related protein: biochemical and biological implications.
|
| |
Protein Sci,
15,
1735-1744.
|
 |
|
PDB code:
|
 |
|
|
|
|
|
 |
N.R.Silvaggi,
C.Zhang,
Z.Lu,
J.Dai,
D.Dunaway-Mariano,
and
K.N.Allen
(2006).
The X-ray crystal structures of human alpha-phosphomannomutase 1 reveal the structural basis of congenital disorder of glycosylation type 1a.
|
| |
J Biol Chem,
281,
14918-14926.
|
 |
|
PDB codes:
|
 |
|
|
|
|
|
 |
P.L.Ipata,
and
M.G.Tozzi
(2006).
Recent advances in structure and function of cytosolic IMP-GMP specific 5'-nucleotidase II (cN-II).
|
| |
Purinergic Signal,
2,
669-675.
|
 |
|
|
|
|
 |
P.R.Beassoni,
L.H.Otero,
M.J.Massimelli,
A.T.Lisa,
and
C.E.Domenech
(2006).
Critical active-site residues identified by site-directed mutagenesis in Pseudomonas aeruginosa phosphorylcholine phosphatase, a new member of the haloacid dehalogenases hydrolase superfamily.
|
| |
Curr Microbiol,
53,
534-539.
|
 |
|
|
|
|
 |
S.D.Lahiri,
G.Zhang,
D.Dunaway-Mariano,
and
K.N.Allen
(2006).
Diversification of function in the haloacid dehalogenase enzyme superfamily: The role of the cap domain in hydrolytic phosphoruscarbon bond cleavage.
|
| |
Bioorg Chem,
34,
394-409.
|
 |
|
PDB codes:
|
 |
|
|
|
|
|
 |
A.Roberts,
S.Y.Lee,
E.McCullagh,
R.E.Silversmith,
and
D.E.Wemmer
(2005).
YbiV from Escherichia coli K12 is a HAD phosphatase.
|
| |
Proteins,
58,
790-801.
|
 |
|
PDB codes:
|
 |
|
|
|
|
|
 |
H.Wang,
H.Pang,
Y.Ding,
Y.Li,
X.Wu,
and
Z.Rao
(2005).
Purification, crystallization and preliminary X-ray diffraction analysis of human enolase-phosphatase E1.
|
| |
Acta Crystallogr Sect F Struct Biol Cryst Commun,
61,
521-523.
|
 |
|
|
|
|
 |
M.Toustrup-Jensen,
and
B.Vilsen
(2005).
Interaction between the catalytic site and the A-M3 linker stabilizes E2/E2P conformational states of Na+,K+-ATPase.
|
| |
J Biol Chem,
280,
10210-10218.
|
 |
|
|
|
|
 |
A.M.Ahmed,
and
T.Shimamoto
(2004).
A plasmid-encoded class 1 integron carrying sat, a putative phosphoserine phosphatase gene and aadA2 from enterotoxigenic Escherichia coli O159 isolated in Japan.
|
| |
FEMS Microbiol Lett,
235,
243-248.
|
 |
|
|
|
|
 |
C.Toyoshima,
and
G.Inesi
(2004).
Structural basis of ion pumping by Ca2+-ATPase of the sarcoplasmic reticulum.
|
| |
Annu Rev Biochem,
73,
269-292.
|
 |
|
|
|
|
 |
C.Toyoshima,
H.Nomura,
and
T.Tsuda
(2004).
Lumenal gating mechanism revealed in calcium pump crystal structures with phosphate analogues.
|
| |
Nature,
432,
361-368.
|
 |
|
PDB codes:
|
 |
|
|
|
|
|
 |
C.Toyoshima,
and
T.Mizutani
(2004).
Crystal structure of the calcium pump with a bound ATP analogue.
|
| |
Nature,
430,
529-535.
|
 |
|
PDB code:
|
 |
|
|
|
|
|
 |
D.B.McIntosh,
J.D.Clausen,
D.G.Woolley,
D.H.MacLennan,
B.Vilsen,
and
J.P.Andersen
(2004).
Roles of conserved P domain residues and Mg2+ in ATP binding in the ground and Ca2+-activated states of sarcoplasmic reticulum Ca2+-ATPase.
|
| |
J Biol Chem,
279,
32515-32523.
|
 |
|
|
|
|
 |
D.H.Shin,
Y.Lou,
J.Jancarik,
H.Yokota,
R.Kim,
and
S.H.Kim
(2004).
Crystal structure of YjeQ from Thermotoga maritima contains a circularly permuted GTPase domain.
|
| |
Proc Natl Acad Sci U S A,
101,
13198-13203.
|
 |
|
PDB code:
|
 |
|
|
|
|
|
 |
E.C.Meng,
B.J.Polacco,
and
P.C.Babbitt
(2004).
Superfamily active site templates.
|
| |
Proteins,
55,
962-976.
|
 |
|
|
|
|
 |
G.Inesi,
H.Ma,
D.Lewis,
and
C.Xu
(2004).
Ca2+ occlusion and gating function of Glu309 in the ADP-fluoroaluminate analog of the Ca2+-ATPase phosphoenzyme intermediate.
|
| |
J Biol Chem,
279,
31629-31637.
|
 |
|
|
|
|
 |
H.Zhu,
S.Yin,
and
S.Shuman
(2004).
Characterization of polynucleotide kinase/phosphatase enzymes from Mycobacteriophages omega and Cjw1 and vibriophage KVP40.
|
| |
J Biol Chem,
279,
26358-26369.
|
 |
|
|
|
|
 |
J.D.Clausen,
B.Vilsen,
D.B.McIntosh,
A.P.Einholm,
and
J.P.Andersen
(2004).
Glutamate-183 in the conserved TGES motif of domain A of sarcoplasmic reticulum Ca2+-ATPase assists in catalysis of E2/E2P partial reactions.
|
| |
Proc Natl Acad Sci U S A,
101,
2776-2781.
|
 |
|
|
|
|
 |
S.Allegrini,
A.Scaloni,
M.G.Careddu,
G.Cuccu,
C.D'Ambrosio,
R.Pesi,
M.Camici,
L.Ferrara,
and
M.G.Tozzi
(2004).
Mechanistic studies on bovine cytosolic 5'-nucleotidase II, an enzyme belonging to the HAD superfamily.
|
| |
Eur J Biochem,
271,
4881-4891.
|
 |
|
|
|
|
 |
S.Danko,
K.Yamasaki,
T.Daiho,
and
H.Suzuki
(2004).
Distinct natures of beryllium fluoride-bound, aluminum fluoride-bound, and magnesium fluoride-bound stable analogues of an ADP-insensitive phosphoenzyme intermediate of sarcoplasmic reticulum Ca2+-ATPase: changes in catalytic and transport sites during phosphoenzyme hydrolysis.
|
| |
J Biol Chem,
279,
14991-14998.
|
 |
|
|
|
|
 |
S.Hausmann,
H.Erdjument-Bromage,
and
S.Shuman
(2004).
Schizosaccharomyces pombe carboxyl-terminal domain (CTD) phosphatase Fcp1: distributive mechanism, minimal CTD substrate, and active site mapping.
|
| |
J Biol Chem,
279,
10892-10900.
|
 |
|
|
|
|
 |
S.K.Singh,
K.Yang,
S.Karthikeyan,
T.Huynh,
X.Zhang,
M.A.Phillips,
and
H.Zhang
(2004).
The thrH gene product of Pseudomonas aeruginosa is a dual activity enzyme with a novel phosphoserine:homoserine phosphotransferase activity.
|
| |
J Biol Chem,
279,
13166-13173.
|
 |
|
PDB codes:
|
 |
|
|
|
|
|
 |
Y.Kim,
A.F.Yakunin,
E.Kuznetsova,
X.Xu,
M.Pennycooke,
J.Gu,
F.Cheung,
M.Proudfoot,
C.H.Arrowsmith,
A.Joachimiak,
A.M.Edwards,
and
D.Christendat
(2004).
Structure- and function-based characterization of a new phosphoglycolate phosphatase from Thermoplasma acidophilum.
|
| |
J Biol Chem,
279,
517-526.
|
 |
|
PDB code:
|
 |
|
|
|
|
|
 |
Y.Peeraer,
A.Rabijns,
J.F.Collet,
E.Van Schaftingen,
and
C.De Ranter
(2004).
How calcium inhibits the magnesium-dependent enzyme human phosphoserine phosphatase.
|
| |
Eur J Biochem,
271,
3421-3427.
|
 |
|
|
|
|
 |
C.Toyoshima,
H.Nomura,
and
Y.Sugita
(2003).
Crystal structures of Ca2+-ATPase in various physiological states.
|
| |
Ann N Y Acad Sci,
986,
1-8.
|
 |
|
|
|
|
 |
D.H.Shin,
A.Roberts,
J.Jancarik,
H.Yokota,
R.Kim,
D.E.Wemmer,
and
S.H.Kim
(2003).
Crystal structure of a phosphatase with a unique substrate binding domain from Thermotoga maritima.
|
| |
Protein Sci,
12,
1464-1472.
|
 |
|
PDB code:
|
 |
|
|
|
|
|
 |
D.L.Stokes,
and
N.M.Green
(2003).
Structure and function of the calcium pump.
|
| |
Annu Rev Biophys Biomol Struct,
32,
445-468.
|
 |
|
|
|
|
 |
H.Ma,
G.Inesi,
and
C.Toyoshima
(2003).
Substrate-induced conformational fit and headpiece closure in the Ca2+ATPase (SERCA).
|
| |
J Biol Chem,
278,
28938-28943.
|
 |
|
|
|
|
 |
M.Toustrup-Jensen,
and
B.Vilsen
(2003).
Importance of conserved Thr214 in domain A of the Na+,K+ -ATPase for stabilization of the phosphoryl transition state complex in E2P dephosphorylation.
|
| |
J Biol Chem,
278,
11402-11410.
|
 |
|
|
|
|
 |
R.D.Makde,
V.Kumar,
G.D.Gupta,
J.Jasti,
T.P.Singh,
and
S.K.Mahajan
(2003).
Expression, purification, crystallization and preliminary X-ray diffraction studies of recombinant class B non-specific acid phosphatase of Salmonella typhimurium.
|
| |
Acta Crystallogr D Biol Crystallogr,
59,
1849-1852.
|
 |
|
|
|
|
 |
R.E.Silversmith,
G.P.Guanga,
L.Betts,
C.Chu,
R.Zhao,
and
R.B.Bourret
(2003).
CheZ-mediated dephosphorylation of the Escherichia coli chemotaxis response regulator CheY: role for CheY glutamate 89.
|
| |
J Bacteriol,
185,
1495-1502.
|
 |
|
PDB code:
|
 |
|
|
|
|
|
 |
S.Hausmann,
and
S.Shuman
(2003).
Defining the active site of Schizosaccharomyces pombe C-terminal domain phosphatase Fcp1.
|
| |
J Biol Chem,
278,
13627-13632.
|
 |
|
|
|
|
 |
X.Li,
K.A.Oghi,
J.Zhang,
A.Krones,
K.T.Bush,
C.K.Glass,
S.K.Nigam,
A.K.Aggarwal,
R.Maas,
D.W.Rose,
and
M.G.Rosenfeld
(2003).
Eya protein phosphatase activity regulates Six1-Dach-Eya transcriptional effects in mammalian organogenesis.
|
| |
Nature,
426,
247-254.
|
 |
|
|
|
|
 |
Y.Peeraer,
A.Rabijns,
C.Verboven,
J.F.Collet,
E.Van Schaftingen,
and
C.De Ranter
(2003).
High-resolution structure of human phosphoserine phosphatase in open conformation.
|
| |
Acta Crystallogr D Biol Crystallogr,
59,
971-977.
|
 |
|
PDB code:
|
 |
|
|
|
|
|
 |
H.Y.Kim,
Y.S.Heo,
J.H.Kim,
M.H.Park,
J.Moon,
E.Kim,
D.Kwon,
J.Yoon,
D.Shin,
E.J.Jeong,
S.Y.Park,
T.G.Lee,
Y.H.Jeon,
S.Ro,
J.M.Cho,
and
K.Y.Hwang
(2002).
Molecular basis for the local conformational rearrangement of human phosphoserine phosphatase.
|
| |
J Biol Chem,
277,
46651-46658.
|
 |
|
PDB codes:
|
 |
|
|
 |
 |
|
The most recent references are shown first.
Citation data come partly from CiteXplore and partly
from an automated harvesting procedure. Note that this is likely to be
only a partial list as not all journals are covered by
either method. However, we are continually building up the citation data
so more and more references will be included with time.
Where a reference describes a PDB structure, the PDB
codes are
shown on the right.
|
 |

