 |
PDBsum entry 1a8r

|

|

|
|
 |
Contents |
 |
|
|

|

|
|
|
|
|
|
|
|
* Residue conservation analysis
|
|
|

|
|
PDB id:
|
 |
|
 |
| Name: |
 |
Hydrolase
|
 |
|
Title:
|
 |
Gtp cyclohydrolase i (h112s mutant) in complex with gtp
|
|
Structure:
|
 |
Gtp cyclohydrolase i. Chain: a, b, c, d, e, f, g, h, i, j, k, l, m, n, o. Engineered: yes. Mutation: yes
|
|
Source:
|
 |
Escherichia coli. Organism_taxid: 562. Organ: brain. Expressed in: escherichia coli. Expression_system_taxid: 562. Other_details: homologous expression
|
|
Biol. unit:
|
 |
 Homo-Decamer (from PDB file)
Homo-Decamer (from PDB file)
|
|
Resolution:
|
 |
|
2.10Å
|
R-factor:
|
0.200
|
R-free:
|
0.246
|
|
|
Authors:
|
 |
G.Auerbach,H.Nar,A.Bracher,A.Bacher,R.Huber
|
Key ref:

|
 |
J.Rebelo
et al.
(2003).
Biosynthesis of pteridines. Reaction mechanism of GTP cyclohydrolase I.
J Mol Biol,
326,
503-516.
PubMed id:
DOI:

|
 |
|
Date:
|
 |
|
27-Mar-98
|
Release date:
|
11-May-99
|
|
|

|

|
|
PROCHECK
|

|

|
|
|
Headers
|
 |
|
|
References
|
|
|
|

|

|
|
P0A6T5
(GCH1_ECOLI) -
GTP cyclohydrolase 1 from Escherichia coli (strain K12)
|
|

|

|
Seq:
Struc:
|
 |
 |
 |
222 a.a.
221 a.a.*
|

|

|

|

|
|

|

|

|
 |
 |

|
|
Key: |
 |
PfamA domain |
 |
 |
 |
Secondary structure |
 |
 |
CATH domain |
 |
|
*
PDB and UniProt seqs differ
at 1 residue position (black
cross)
|
|

|
|
|
 |
|
|
 |
 |
 |
 |
Enzyme class:
|
 |
E.C.3.5.4.16
- Gtp cyclohydrolase i.
|
|
 |
 |
 |
 |
 |

Pathway:
|
 |
Folate Biosynthesis (early stages)
|
 |
 |
 |
 |
 |
Reaction:
|
 |
GTP + H2O = 7,8-dihydroneopterin 3'-triphosphate + formate + H+
|
 |
 |
 |
 |
 |
GTP
Bound ligand (Het Group name = )
corresponds exactly
|
+
|
H2O
|
=
|
7,8-dihydroneopterin 3'-triphosphate
|
+
|
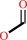 formate
formate
|
+
|
H(+)
|
|
 |
 |
 |
 |
 |
 |
 |
 |
 |
 |
|
Molecule diagrams generated from .mol files obtained from the
KEGG ftp site
|
|
 |
 |
 |
 |
 |
 |
 |
 |
 |
 |
 |
 |
 |
 |
 |

|

|

|
| |
|
|
| |
|
DOI no:
|
J Mol Biol
326:503-516
(2003)
|
|
PubMed id:
|
|
|
|

|
| |
|
Biosynthesis of pteridines. Reaction mechanism of GTP cyclohydrolase I.
|
|
J.Rebelo,
G.Auerbach,
G.Bader,
A.Bracher,
H.Nar,
C.Hösl,
N.Schramek,
J.Kaiser,
A.Bacher,
R.Huber,
M.Fischer.
|
|
|

|
| |
ABSTRACT
|
|

|
| |

|
GTP cyclohydrolase I catalyses the hydrolytic release of formate from GTP
followed by cyclization to dihydroneopterin triphosphate. The enzymes from
bacteria and animals are homodecamers containing one zinc ion per subunit.
Replacement of Cys110, Cys181, His112 or His113 of the enzyme from Escherichia
coli by serine affords catalytically inactive mutant proteins with reduced
capacity to bind zinc. These mutant proteins are unable to convert GTP or the
committed reaction intermediate,
2-amino-5-formylamino-6-(beta-ribosylamino)-4(3H)-pyrimidinone 5'-triphosphate,
to dihydroneopterin triphosphate. The crystal structures of GTP complexes of the
His113Ser, His112Ser and Cys181Ser mutant proteins determined at resolutions of
2.5A, 2.8A and 3.2A, respectively, revealed the conformation of substrate GTP in
the active site cavity. The carboxylic group of the highly conserved residue
Glu152 anchors the substrate GTP, by hydrogen bonding to N-3 and to the position
2 amino group. Several basic amino acid residues interact with the triphosphate
moiety of the substrate. The structure of the His112Ser mutant in complex with
an undefined mixture of nucleotides determined at a resolution of 2.1A afforded
additional details of the peptide folding. Comparison between the wild-type and
mutant enzyme structures indicates that the catalytically active zinc ion is
directly coordinated to Cys110, Cys181 and His113. Moreover, the zinc ion is
complexed to a water molecule, which is in close hydrogen bond contact to
His112. In close analogy to zinc proteases, the zinc-coordinated water molecule
is suggested to attack C-8 of the substrate affording a zinc-bound 8R hydrate of
GTP. Opening of the hydrated imidazole ring affords a formamide derivative,
which remains coordinated to zinc. The subsequent hydrolysis of the formamide
motif has an absolute requirement for zinc ion catalysis. The hydrolysis of the
formamide bond shows close mechanistic similarity with peptide hydrolysis by
zinc proteases.

|

|
|
|

|
| |
Selected figure(s)
|
|

|
| |
 |
 |

|
 |

|
 |
Figure 1.
Figure 1. Hypothetical mechanism of the reaction catalysed by
GTP cyclohydrolase I.[8.]
|
 |
Figure 4.
Figure 4. Stereo diagram from the active site of the E. coli
GTP cyclohydrolase I His113Ser mutant in complex with the
substrate GTP. The GTP molecule (shown as a transparent wire
model representation) is embedded in a large hydrogen bond
network (broken lines) within the active site. Amino acid
residues are shown as ball-and-stick models coloured according
to the subunit to which they belong: A, red; B, blue; and D,
green. The Figure was created using MOLSCRIPT[39.] and Raster3D.
[40.]
|
 |
|
|

|
| |
The above figures are
reprinted
by permission from Elsevier:
J Mol Biol
(2003,
326,
503-516)
copyright 2003.
|
|
| |
Figures were
selected
by an automated process.
|
|

|

|
|
 |
 |
|
 |
 |
 |
 |
 |
 |
 |
 |
 |
|
Literature references that cite this PDB file's key reference
|
|
 |
| |
PubMed id
|
 |
Reference
|
 |
|
|
|
 |
B.Sankaran,
S.A.Bonnett,
K.Shah,
S.Gabriel,
R.Reddy,
P.Schimmel,
D.A.Rodionov,
V.de Crécy-Lagard,
J.D.Helmann,
D.Iwata-Reuyl,
and
M.A.Swairjo
(2009).
Zinc-independent folate biosynthesis: genetic, biochemical, and structural investigations reveal new metal dependence for GTP cyclohydrolase IB.
|
| |
J Bacteriol,
191,
6936-6949.
|
 |
|
PDB codes:
|
 |
|
|
|
|
|
 |
S.E.Gibbons,
I.Stayton,
and
Y.Ma
(2009).
Optimization of urinary pteridine analysis conditions by CE-LIF for clinical use in early cancer detection.
|
| |
Electrophoresis,
30,
3591-3597.
|
 |
|
|
|
|
 |
R.M.McCarty,
and
V.Bandarian
(2008).
Deciphering deazapurine biosynthesis: pathway for pyrrolopyrimidine nucleosides toyocamycin and sangivamycin.
|
| |
Chem Biol,
15,
790-798.
|
 |
|
|
|
|
 |
A.Tazumi,
S.Saito,
T.Sekizuka,
O.Murayama,
J.E.Moore,
B.C.Millar,
and
M.Matsuda
(2007).
Molecular characterization of the non-coding promoter and leader regions and full-length 16S ribosomal RNA (rRNA) gene of Taylorella asinigenitalis.
|
| |
J Basic Microbiol,
47,
260-265.
|
 |
|
|
|
|
 |
B.Nocek,
E.Evdokimova,
M.Proudfoot,
M.Kudritska,
L.L.Grochowski,
R.H.White,
A.Savchenko,
A.F.Yakunin,
A.Edwards,
and
A.Joachimiak
(2007).
Structure of an amide bond forming F(420):gamma-glutamyl ligase from Archaeoglobus fulgidus -- a member of a new family of non-ribosomal peptide synthases.
|
| |
J Mol Biol,
372,
456-469.
|
 |
|
PDB codes:
|
 |
|
|
|
|
|
 |
W.Martin,
and
M.J.Russell
(2007).
On the origin of biochemistry at an alkaline hydrothermal vent.
|
| |
Philos Trans R Soc Lond B Biol Sci,
362,
1887-1925.
|
 |
|
|
|
|
 |
B.Chavan,
J.M.Gillbro,
H.Rokos,
and
K.U.Schallreuter
(2006).
GTP cyclohydrolase feedback regulatory protein controls cofactor 6-tetrahydrobiopterin synthesis in the cytosol and in the nucleus of epidermal keratinocytes and melanocytes.
|
| |
J Invest Dermatol,
126,
2481-2489.
|
 |
|
|
|
|
 |
B.El Yacoubi,
S.Bonnett,
J.N.Anderson,
M.A.Swairjo,
D.Iwata-Reuyl,
and
V.de Crécy-Lagard
(2006).
Discovery of a new prokaryotic type I GTP cyclohydrolase family.
|
| |
J Biol Chem,
281,
37586-37593.
|
 |
|
|
|
|
 |
I.Tegeder,
M.Costigan,
R.S.Griffin,
A.Abele,
I.Belfer,
H.Schmidt,
C.Ehnert,
J.Nejim,
C.Marian,
J.Scholz,
T.Wu,
A.Allchorne,
L.Diatchenko,
A.M.Binshtok,
D.Goldman,
J.Adolph,
S.Sama,
S.J.Atlas,
W.A.Carlezon,
A.Parsegian,
J.Lötsch,
R.B.Fillingim,
W.Maixner,
G.Geisslinger,
M.B.Max,
and
C.J.Woolf
(2006).
GTP cyclohydrolase and tetrahydrobiopterin regulate pain sensitivity and persistence.
|
| |
Nat Med,
12,
1269-1277.
|
 |
|
|
|
|
 |
P.Hänzelmann,
and
H.Schindelin
(2006).
Binding of 5'-GTP to the C-terminal FeS cluster of the radical S-adenosylmethionine enzyme MoaA provides insights into its mechanism.
|
| |
Proc Natl Acad Sci U S A,
103,
6829-6834.
|
 |
|
PDB codes:
|
 |
|
|
|
|
|
 |
J.Ren,
M.Kotaka,
M.Lockyer,
H.K.Lamb,
A.R.Hawkins,
and
D.K.Stammers
(2005).
GTP cyclohydrolase II structure and mechanism.
|
| |
J Biol Chem,
280,
36912-36919.
|
 |
|
PDB codes:
|
 |
|
|
|
|
|
 |
M.Fischer,
and
A.Bacher
(2005).
Biosynthesis of flavocoenzymes.
|
| |
Nat Prod Rep,
22,
324-350.
|
 |
|
|
|
|
 |
S.G.Van Lanen,
J.S.Reader,
M.A.Swairjo,
V.de Crécy-Lagard,
B.Lee,
and
D.Iwata-Reuyl
(2005).
From cyclohydrolase to oxidoreductase: discovery of nitrile reductase activity in a common fold.
|
| |
Proc Natl Acad Sci U S A,
102,
4264-4269.
|
 |
|
|
|
|
 |
G.Bader,
M.Gomez-Ortiz,
C.Haussmann,
A.Bacher,
R.Huber,
and
M.Fischer
(2004).
Structure of the molybdenum-cofactor biosynthesis protein MoaB of Escherichia coli.
|
| |
Acta Crystallogr D Biol Crystallogr,
60,
1068-1075.
|
 |
|
PDB code:
|
 |
|
|
|
|
|
 |
L.I.Leichert,
and
U.Jakob
(2004).
Protein thiol modifications visualized in vivo.
|
| |
PLoS Biol,
2,
e333.
|
 |
|
|
|
|
 |
M.A.Kolinsky,
and
S.S.Gross
(2004).
The mechanism of potent GTP cyclohydrolase I inhibition by 2,4-diamino-6-hydroxypyrimidine: requirement of the GTP cyclohydrolase I feedback regulatory protein.
|
| |
J Biol Chem,
279,
40677-40682.
|
 |
|
|
|
|
 |
P.Hänzelmann,
H.L.Hernández,
C.Menzel,
R.García-Serres,
B.H.Huynh,
M.K.Johnson,
R.R.Mendel,
and
H.Schindelin
(2004).
Characterization of MOCS1A, an oxygen-sensitive iron-sulfur protein involved in human molybdenum cofactor biosynthesis.
|
| |
J Biol Chem,
279,
34721-34732.
|
 |
|
|
|
|
 |
T.Suzuki,
H.Kurita,
and
H.Ichinose
(2004).
GTP cyclohydrolase I utilizes metal-free GTP as its substrate.
|
| |
Eur J Biochem,
271,
349-355.
|
 |
|
|
|
|
 |
A.He,
and
J.P.Rosazza
(2003).
GTP cyclohydrolase I: purification, characterization, and effects of inhibition on nitric oxide synthase in nocardia species.
|
| |
Appl Environ Microbiol,
69,
7507-7513.
|
 |
|
 |
 |
|
The most recent references are shown first.
Citation data come partly from CiteXplore and partly
from an automated harvesting procedure. Note that this is likely to be
only a partial list as not all journals are covered by
either method. However, we are continually building up the citation data
so more and more references will be included with time.
Where a reference describes a PDB structure, the PDB
codes are
shown on the right.
|
 |

