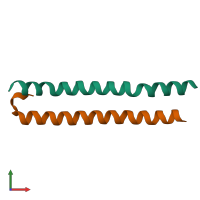Assemblies
Assembly Name:
Serine/threonine-protein kinase 4 18kDa subunit and Ras association domain-containing protein 5
Multimeric state:
hetero dimer
Accessible surface area:
6765.56 Å2
Buried surface area:
2402.46 Å2
Dissociation area:
1,201.23
Å2
Dissociation energy (ΔGdiss):
14.62
kcal/mol
Dissociation entropy (TΔSdiss):
9.96
kcal/mol
Symmetry number:
1
PDBe Complex ID:
PDB-CPX-171514
Macromolecules
Chain: A
Length: 51 amino acids
Theoretical weight: 6.12 KDa
Source organism: Homo sapiens
Expression system: Escherichia coli BL21
UniProt:
Pfam: C terminal SARAH domain of Mst1
InterPro:
CATH: p53-like tetramerisation domain
Length: 51 amino acids
Theoretical weight: 6.12 KDa
Source organism: Homo sapiens
Expression system: Escherichia coli BL21
UniProt:
- Canonical:
 Q13043 (Residues: 432-480; Coverage: 10%)
Q13043 (Residues: 432-480; Coverage: 10%)
Pfam: C terminal SARAH domain of Mst1
InterPro:
CATH: p53-like tetramerisation domain
Chain: B
Length: 55 amino acids
Theoretical weight: 6.52 KDa
Source organism: Homo sapiens
Expression system: Escherichia coli BL21
UniProt:
Pfam: Novel Ras effector 1 C-terminal SARAH (Sav/Rassf/Hpo) domain
InterPro:
CATH: Single alpha-helices involved in coiled-coils or other helix-helix interfaces
Length: 55 amino acids
Theoretical weight: 6.52 KDa
Source organism: Homo sapiens
Expression system: Escherichia coli BL21
UniProt:
- Canonical:
 Q8WWW0 (Residues: 366-418; Coverage: 13%)
Q8WWW0 (Residues: 366-418; Coverage: 13%)
Pfam: Novel Ras effector 1 C-terminal SARAH (Sav/Rassf/Hpo) domain
InterPro:
CATH: Single alpha-helices involved in coiled-coils or other helix-helix interfaces