Assemblies
Assembly Name:
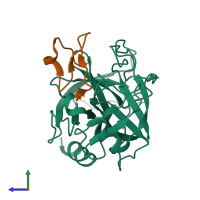
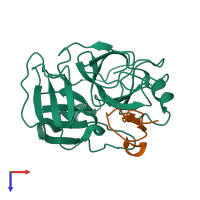
Serine protease 1 and Trypsin inhibitor 1
Multimeric state:
hetero dimer
Accessible surface area:
9918.74 Å2
Buried surface area:
1684.38 Å2
Dissociation area:
842.19
Å2
Dissociation energy (ΔGdiss):
5.5
kcal/mol
Dissociation entropy (TΔSdiss):
9.3
kcal/mol
Symmetry number:
1
PDBe Complex ID:
PDB-CPX-133328
Macromolecules
Chain: E
Length: 223 amino acids
Theoretical weight: 23.32 KDa
Source organism: Bos taurus
Expression system: Not provided
UniProt:
Pfam: Trypsin
InterPro:
SCOP: Eukaryotic proteases
Length: 223 amino acids
Theoretical weight: 23.32 KDa
Source organism: Bos taurus
Expression system: Not provided
UniProt:
- Canonical:
 P00760 (Residues: 24-246; Coverage: 97%)
P00760 (Residues: 24-246; Coverage: 97%)
Pfam: Trypsin
InterPro:
- Serine proteases, trypsin domain
- Peptidase S1, PA clan
- Peptidase S1, PA clan, chymotrypsin-like fold
- Peptidase S1A, chymotrypsin family
- Serine proteases, trypsin family, histidine active site
- Serine proteases, trypsin family, serine active site
SCOP: Eukaryotic proteases
Chain: I
Length: 29 amino acids
Theoretical weight: 3.28 KDa
Source organism: Cucurbita maxima
Expression system: Not provided
UniProt:
InterPro:
SCOP: Plant inhibitors of proteinases and amylases
Length: 29 amino acids
Theoretical weight: 3.28 KDa
Source organism: Cucurbita maxima
Expression system: Not provided
UniProt:
- Canonical:
 P01074 (Residues: 1-29; Coverage: 100%)
P01074 (Residues: 1-29; Coverage: 100%)
InterPro:
SCOP: Plant inhibitors of proteinases and amylases