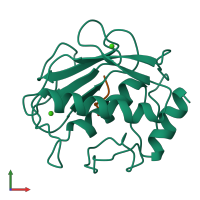
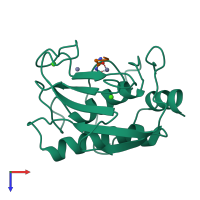
Function and Biology Details
Reaction catalysed:
Cleavage of interstitial collagens in the triple helical domain. Unlike EC 3.4.24.7, this enzyme cleaves type III collagen more slowly than type I.
Biochemical function:
Biological process:
Cellular component:
Structure analysis Details
Assembly composition:
hetero dimer (preferred)
Assembly name:
Neutrophil collagenase and peptide (preferred)
PDBe Complex ID:
PDB-CPX-149833 (preferred)
Entry contents:
2 distinct polypeptide molecules
Macromolecules (2 distinct):