Teusink et al., (1998). The danger of metabolic pathways with turbo design.
February 2013, model of the month by Benedetta Frida Baldi
Original model: BIOMD0000000253
"Substrate accelerated death" is a phenomenon that occurs when starved bacteria are exposed to the substrate, which limits their growth [1]. This phenomenon has been observed in both, prokaryotes and eukaryotes like Saccharomyces cerevisiae, and could be related to the "turbo design" of metabolic pathways. A turbo engine creates motion by mixing air with fuel to create expanding and fast moving exhaust gases that are blasted out. The hot exhaust gases are then fed back into the engine to accelerate the fuel input. A biological example of this principle is the glycolytic pathway. Glycolysis is a catabolic pathway consisting of several steps in S.cerevisiae leading to the conversion of glucose to ethanol. A complete picture of the pathway can be found in the KEGG pathway database. A more generic representation is shown in Figure 1. The pathway can be divided into two main blocks: The first ATP activated path produces intermediates coupled with the hydrolysis of 2 molecules of ATP, the second one produces 4 molecules of ATP (Figure 2).
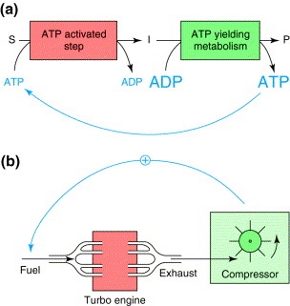
Figure 2 Comparison of turbo designs. a) General scheme of the glycolytic pathway in which the first step is coupled to ATP hydrolysis for the conversion of a substrate S in an intermediate I. In the second step, intermediates are converted to a product P with generation of a surplus of ATP. b) Schematic representation of a turbo engine, in which exhaust gases are used to increase the influx of fuel. Figure taken from [3].
Teusink and co-workers [3, BIOMD0000000253] use a deterministic modelling approach to show the crucial importance of this inhibitory step. The model implemented here contains a set of 4 reactions (Figure 3). The first reaction is the HK mediated conversion of glucose to glucose monophosphate coupled to the hydrolysis of ATP. In the second reaction, the phosphofructokinase (PFK) converts glucose and other hexose monophosphate (HMP) to fructose 1,6 bi-phosphate (Fru1,6P2). This step is also coupled to ATP consumption. PFK was modelled as Monod-Wyman-Changeux enzyme with HMP and ATP as substrates. The other subsequent reactions of glycolysis were condensed into one step, leading to the production of 2 molecules of ethanol (EtOH) and 4 ATPs. The last reaction is a general ATPase reaction. The inhibition of the hexokinase was modelled as competitive inhibition. The concentration of Tre6P was calculated with a simple quadratic relationship with the HMP concentration due to the lack of experimental data.

Figure 3 Schematic representation of the core model of glycolysis. In the model the lower part of glycolysis is represented as a single step. Glc, glucose; HMP, hexose monophosphate; Fru 1,6-P2, fructose 1,6-bisphosphate; Tre 6-P, trehalose 6-phosphate; EtOH, ethanol; HK, hexokinase; PFK, phosphofructokinase; Tps1p, Tre 6-P synthase; lower, lower part of glycolysis. Figure taken from [3].

Figure 6 Simulation time course of the model (Figure 3) with the negative feedback on HK. The initial concentrations of hexose monophosphate (HMP), fructose 1,6-bisphosphate (Fru 1,6-P2) and ATP were 0.1 mm, 1 mm and 4.0 mm, respectively. νHK, rate of the hexokinase reaction; νPFK, rate of the phosphofructokinase reaction; νlower, rate of the lower part of glycolysis; νATPase, rate of the ATPase reaction. Figure taken from [3].

Figure 1 Cartoon representation of the glycolytic pathway. Figure taken from [2].
Mutations in the TPS1 gene in S.cerevisiae gene, which encodes for trehalose 6-phosphate (Tre6P) synthase, always results in the loss of the ability to grow on glucose. Surprisingly, Tre6P synthase is not directly involved in the catabolism of glucose. The product of Tre6P synthase – Tre6P - is known to inhibit the first step of glycolysis. The hexokinase (HK) is not directly inhibited by its product glucose monophosphate or ADP in S.cerevisiae unlike other organisms, but susceptible to feedback inhibition by trehalose 6-phosphate.

Figure 4 Simulation time course of the model (Figure 3) without the negative feedback on HK. ATP falls into a steady state while intermediates accumulate in the cell. The initial concentrations of hexose monophosphate (HMP), fructose 1,6-bisphosphate (Fru 1,6-P2) and ATP were 0.1 mm, 1 mm and 4.0 mm, respectively. νHK, rate of the hexokinase reaction; νPFK, rate of the phosphofructokinase reaction; νlower, rate of the lower part of glycolysis; νATPase, rate of the ATPase reaction. Figure taken from [3].
An interesting analysis is the performance of the model in the absence of negative feedback. In this condition, the pathway fails to produce a sufficient amount of ATP, while intermediates such as HMP and Fru1, 6P2 accumulate in the cell (Figure 4). Moreover, ATP seems to reach a quasi steady state, while hexose phosphates do not. In the absence of Tre6P inhibition, ATP is the only regulatory feedback present in the pathway. This regulation becomes insufficient as soon as the ATP concentration tunes the ATP production to the ATP consumption. As soon as ATP reaches a steady state it is no longer able to influence the hexose phosphate production and degradation. A complete steady state as in case of the feedback inhibition can only be reached by reducing the hexokinase activity to 20% but the levels of ATP and Fru1,6-P2 are lower (Figure 5-6).

Figure 5 Simulation time course of the model (Figure 3) without the negative feedback and with HK activity reduced to its 20%. The inhibition of HK led restoration of a complete steady state (both ATP and intermediates). The initial concentrations of hexose monophosphate (HMP), fructose 1,6-bisphosphate (Fru 1,6-P2) and ATP were 0.1 mm, 1 mm and 4.0 mm, respectively. νHK, rate of the hexokinase reaction; νPFK, rate of the phosphofructokinase reaction; νlower, rate of the lower part of glycolysis; νATPase, rate of the ATPase reaction. Figure taken from [3].
As mentioned above, other organisms differ from S.cerevisiae because they show direct inhibition of the hexokinase. The authors speculate that the TPS1 negative feedback has been an evolutionary selection to cope with rapid changes in environmental glucose concentrations. Unicellular organisms need to adapt quickly to different external conditions such as nutrient concentrations unlike mammalian systems that can rely on efficient homeostatic controls.
If the substrates become suddenly available in excess, this turbo pathway design becomes extremely vulnerable. The flaw of this turbo design lies in the activating step of the glycolysis. This first step is thermodynamically irreversible, thanks to its coupling to ATP hydrolysis and for this reason it is not subjected to mass action law control. In case of excess substrate, the second part of the pathway becomes a bottleneck leading to accumulation of intermediates. Inhibition through an external effector such as TPS1 introduces an extra layer of regulation therefore increasing the dynamic range of the inhibition under variable external conditions.
The authors explain this "substrate accelerated death" phenomenon through the absence of a specific regulatory mechanism, which prevents activated pathways with turbo design to adapt to the environmental conditions.
Bibliographic references
- Postgate, JR & Hunter, JR. Acceleration of bacterial death by growth substrates. Nature. 1963, Apr 20;198:273.
- Michels, PA. Evolutionary aspects of trypanosomes: analysis of genes. J Mol Evol. 1986;24(1-2):45-52.
- Teusink et al. The danger of metabolic pathways with turbo design. Trends Biochem Sci. 1998, May;23(5):162-9.
