Phosphoglycerate kinase
The first energy-producing reaction of glycolysis is catalysed by phosphoglycerate kinase (PGK). This enzyme is unusual among kinases in that, biologically it functions mainly in the reverse (with respect to the EC) direction to phosphorylate ADP. Here we show the biological direction. This important energy saving feature makes it fairly ubiquitous. In all known cases it requires an Mg(II) ion to reduce charge repulsion between the negatively charged phosphate groups, enabling the two charged phosphate groups to interact.
Reference Protein and Structure
- Sequence
-
P07378
 (2.7.2.3)
(2.7.2.3)
 (Sequence Homologues)
(PDB Homologues)
(Sequence Homologues)
(PDB Homologues)
- Biological species
-
Trypanosoma brucei brucei (Trypanosome)

- PDB
-
13pk
- TERNARY COMPLEX OF PHOSPHOGLYCERATE KINASE FROM TRYPANOSOMA BRUCEI
(2.5 Å)



- Catalytic CATH Domains
-
3.40.50.1260
 (see all for 13pk)
(see all for 13pk)
- Cofactors
- Magnesium(2+) (1) Metal MACiE
Enzyme Reaction (EC:2.7.2.3)
Enzyme Mechanism
Introduction
Phosphoglycerate kinase catalyses the reversible phosphoryl transfer between 1,3-bisphosphoglycerate and ADP to form 3-phosphoglycerate and ATP, in the presence of magnesium. Phosphoryl transfer occurs by a single step in-line mechanism, or SN2-reaction mechanism, with nucleophilic attack by the ADP-beta-phosphate oxygen atom at the 1-phosphate group of 1,3-BPG. This reaction involves an inversion of configuration at the gamma phosphorous atom. The main role of the enzyme is to orientate the two compounds favourably with respect to each other and to stabilise the pentacoordinate transition state. The protein undergoes a conformational change after binding substrate to exclude water from the active site; in the closed formation, the Lys219 side chain moves into position to assist the main chain amides of two residues, Gly376 and Gly399, the Mg(II) ion and the Arg39 side chain in transition state stabilisation.
Catalytic Residues Roles
| UniProt | PDB* (13pk) | ||
| Lys219 | Lys219(215)A | Stabilises phosphoryl group oxygen atom in transition state. | hydrogen bond donor, electrostatic stabiliser |
| Gly399 (main-N), Gly376 (main-N), Arg39 | Gly399(395)A (main-N), Gly376(372)A (main-N), Arg39(35)A | Activates and stabilises phosphoryl group oxygen atom in transition state. | hydrogen bond donor, electrostatic stabiliser |
Chemical Components
bimolecular nucleophilic substitution, overall reactant used, overall product formed, rate-determining stepReferences
- Bernstein BE et al. (1998), Biochemistry, 37, 4429-4436. Crystal Structures of Substrates and Products Bound to the Phosphoglycerate Kinase Active Site Reveal the Catalytic Mechanism†. DOI:10.1021/bi9724117. PMID:9521762.
- Vas M et al. (2008), Moscow University Chemistry Bulletin, 63, 114-119. Insight into the mechanism of domain movements and its role in functioning of 3-phosphoglycerate kinase. DOI:10.3103/s0027131408020156.
- Varga A et al. (2005), FEBS J, 272, 1867-1885. Correlation between conformational stability of the ternary enzyme-substrate complex and domain closure of 3-phosphoglycerate kinase. DOI:10.1111/j.1742-4658.2005.04618.x. PMID:15819882.
- Flachner B et al. (2004), Biochemistry, 43, 3436-3449. Role of Phosphate Chain Mobility of MgATP in Completing the 3-Phosphoglycerate Kinase Catalytic Site: Binding, Kinetic, and Crystallographic Studies with ATP and MgATP†. DOI:10.1021/bi035022n. PMID:15035615.
- Bressi JC et al. (2000), J Med Chem, 43, 4135-4150. Adenosine Analogues as Inhibitors ofTrypanosomabruceiPhosphoglycerate Kinase: Elucidation of a Novel Binding Mode for a 2-Amino-N6-Substituted Adenosine. DOI:10.1021/jm000287a.
- Bernstein BE et al. (1998), J Mol Biol, 279, 1137-1148. A bisubstrate analog induces unexpected conformational changes in phosphoglycerate kinase from Trypanosoma brucei. DOI:10.1006/jmbi.1998.1835. PMID:9642090.
- Auerbach G et al. (1997), Structure, 5, 1475-1483. Closed structure of phosphoglycerate kinase from Thermotoga maritima reveals the catalytic mechanism and determinants of thermal stability. DOI:10.1016/s0969-2126(97)00297-9. PMID:9384563.
- Bernstein BE et al. (1997), Nature, 385, 275-278. Synergistic effects of substrate-induced conformational changes in phosphoglycerate kinase activation. DOI:10.1038/385275a0. PMID:9000079.
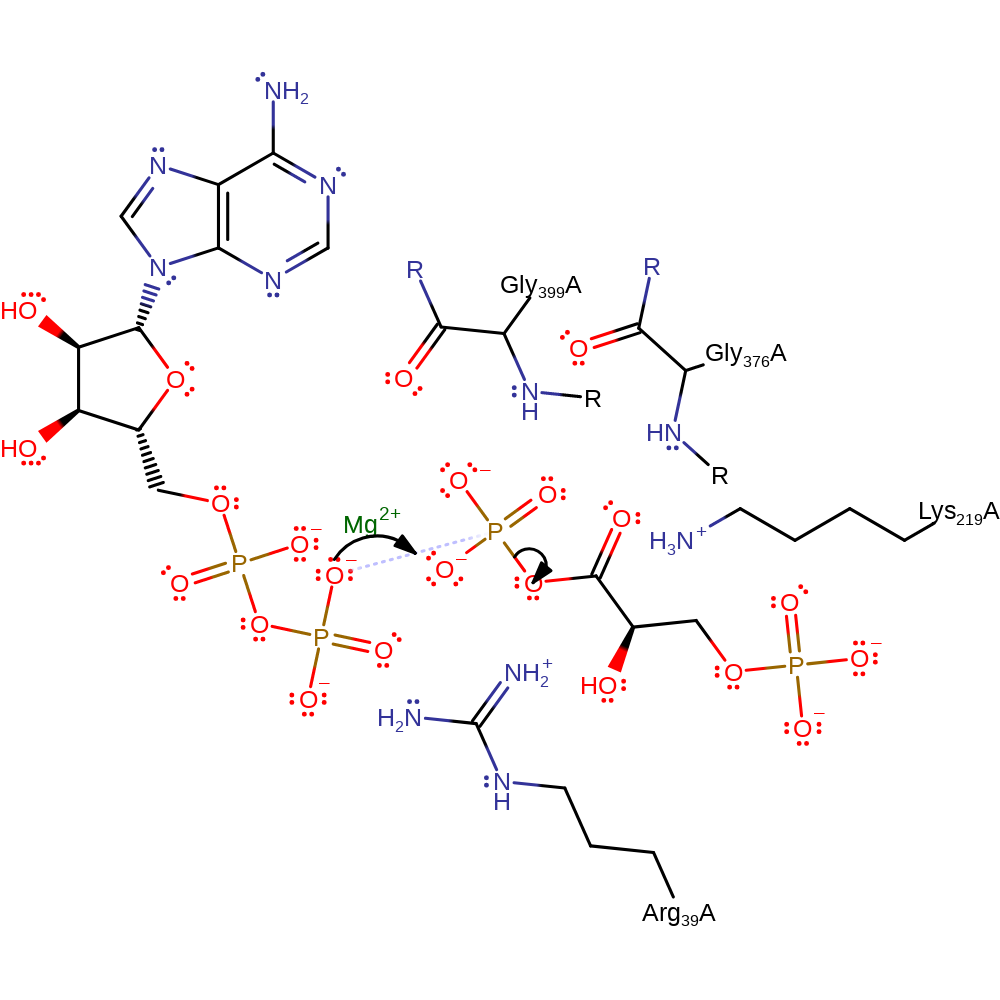
Step 1. The beta-phosphate of ADP initiates a nucleophilic attack on the 1-phosphate of 1,3-biphosphoglycerate in a substitution reaction, eliminating 3-phosphoglycate.
Download: Image, Marvin FileCatalytic Residues Roles
| Residue | Roles |
|---|---|
| Arg39(35)A | electrostatic stabiliser, hydrogen bond donor |
| Lys219(215)A | hydrogen bond donor, electrostatic stabiliser |
| Gly376(372)A (main-N) | electrostatic stabiliser, hydrogen bond donor |
| Gly399(395)A (main-N) | hydrogen bond donor, electrostatic stabiliser |




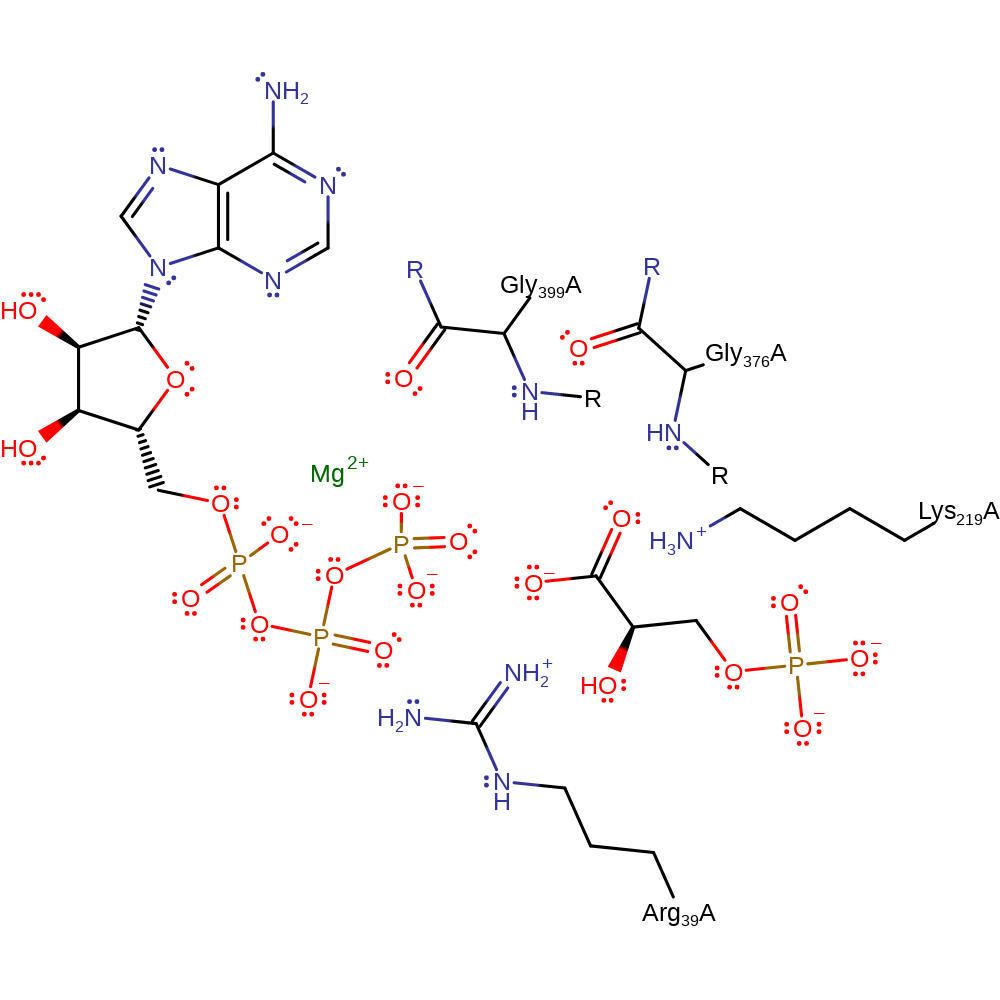 Download:
Download: