Formyl-CoA transferase
Formyl-CoA transferase, sourced from Oxalobacter formigenes colonises the human gastrointestinal tract. It breaks down oxalate to generate ATP. Formyl-CoA transferase is a critical enzyme in oxalate-dependent ATP synthesis. It catalyses the transfer of CoA from formyl-CoA to oxalate, producing oxalyl-CoA and formate. It is of interest due to a correlation between absence of O. formigenes in humans and kidney stone formation due to elevated levels of oxalate in the blood. Secondly ATP production appears to depend solely on the anaerobic conversion of oxalate to formate and carbon dioxide - other carbohydrates cannot be used to replace oxalate as a growth substrate, implying that the organism lacks a functional glycolytic pathway.
Reference Protein and Structure
- Sequence
-
O06644
 (2.8.3.16)
(2.8.3.16)
 (Sequence Homologues)
(PDB Homologues)
(Sequence Homologues)
(PDB Homologues)
- Biological species
-
Oxalobacter formigenes (Bacteria)

- PDB
-
1t4c
- Formyl-CoA Transferase in complex with Oxalyl-CoA
(2.61 Å)



- Catalytic CATH Domains
-
3.30.1540.10
 3.40.50.10540
3.40.50.10540  (see all for 1t4c)
(see all for 1t4c)
Enzyme Reaction (EC:2.8.3.16)
Enzyme Mechanism
Introduction
This mechanism proposal differs from the other in the fact that when CoAS is originally cleaved in the first elimination it then re-attacks the carbonyl carbon so that formate will be eliminated in the next step. Then oxalate will nucleophilically attack Asp169 and CoAS will be cleaved again so that it can re-attack at the carbonyl carbon of oxalate so that Asp169 can be cleaved from the intermediate and release the final product of oxalyl-CoA. This mechanism has been shown to be relevant for Class III CoA-transferases which Formyl-CoA transferase is an example of whereas the previous proposal is an example of class I CoA-transferases (PMID: 18162462)
Catalytic Residues Roles
| UniProt | PDB* (1t4c) | ||
| Glu140 (main-N) | Glu140(139)A (main-N) | Glu 140 stabilises O1 of the oxalyl part of the oxalyl aspartic anhydride through hydrogen bonding to the backbone amide | hydrogen bond donor |
| Gly260 (main-C) | Gly260(259)B (main-C) | Gly 260' stabilises O2 of the oxalyl part of the oxalyl aspartic anhydride through hydrogen bonding to the backbone carbonyl. | hydrogen bond acceptor |
| Gly261 (main-N) | Gly261(260)B (main-N) | Gly 261' stabilises O2 of the oxalyl part of the oxalyl aspartic anhydride through hydrogen bonding to the backbone amide | hydrogen bond donor |
| Gln17 | Gln17(16)A | Gln 17 stabilises O3 of the oxalyl part of the oxalyl aspartic anhydride through hydrogen bonding to the backbone amide. | hydrogen bond donor, electrostatic stabiliser |
| Asp169 | Asp169(168)A | Asp 169 performs nucleophilic attack upon formyl-CoA, attacking the carbonyl group of the thioester. The electrophilic carbonyl group of Asp 169 is nucleophilically attacked by oxalate and CoAS. | covalently attached, nucleofuge, nucleophile, electrofuge, electrophile |
Chemical Components
bimolecular nucleophilic addition, overall reactant used, enzyme-substrate complex formation, intermediate formation, unimolecular elimination by the conjugate base, enzyme-substrate complex cleavage, intermediate collapse, overall product formed, intermediate terminated, native state of enzyme regeneratedReferences
- Berthold CL et al. (2008), J Biol Chem, 283, 6519-6529. Reinvestigation of the catalytic mechanism of formyl-CoA transferase, a class III CoA-transferase. DOI:10.1074/jbc.M709353200. PMID:18162462.

Step 1. Asp169 attacks the carbonyl carbon of formyl-CoA in a nucleophilic addition.
Download: Image, Marvin FileCatalytic Residues Roles
| Residue | Roles |
|---|---|
| Gln17(16)A | hydrogen bond donor, electrostatic stabiliser |
| Glu140(139)A (main-N) | hydrogen bond donor |
| Gly260(259)B (main-C) | hydrogen bond acceptor |
| Gly261(260)B (main-N) | hydrogen bond donor |
| Asp169(168)A | nucleophile |
Chemical Components
ingold: bimolecular nucleophilic addition, overall reactant used, enzyme-substrate complex formation, intermediate formation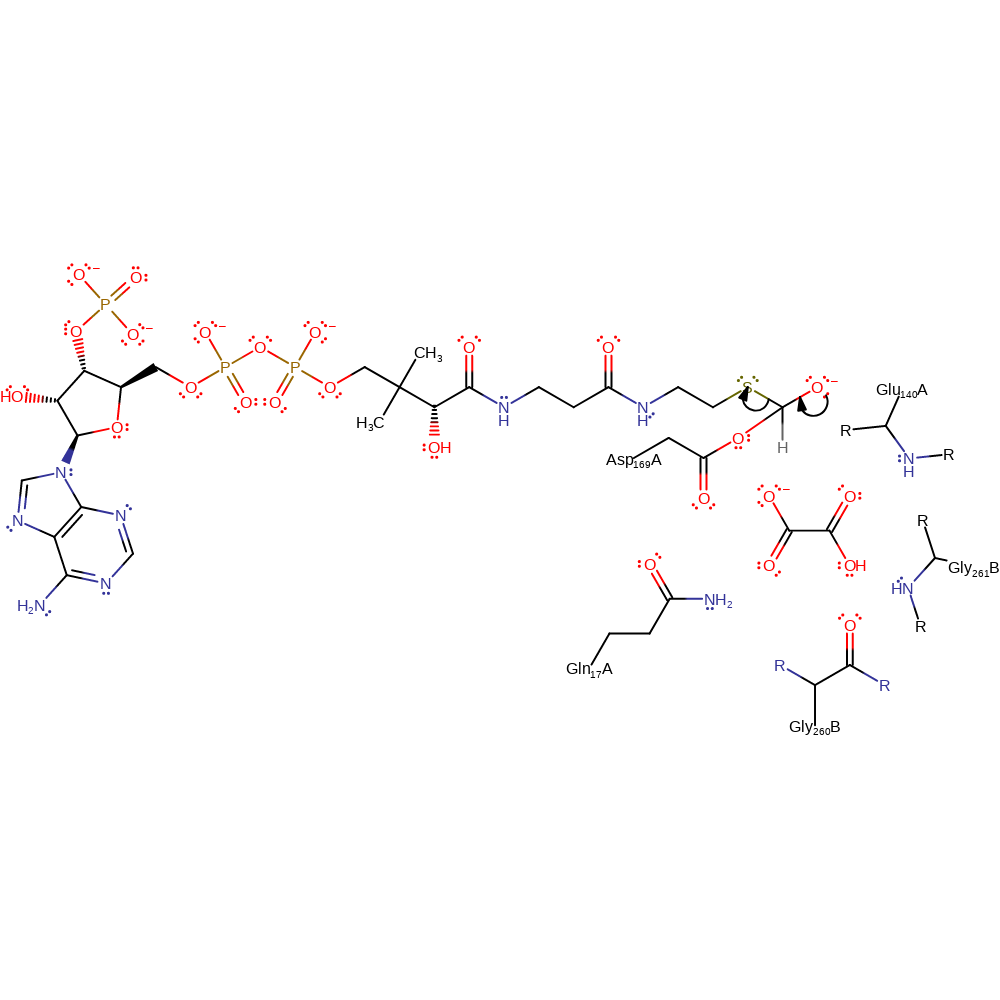
Step 2. The oxyanion initiates an elimination of the CoA, leaving the Asp169 acylated.
Download: Image, Marvin FileCatalytic Residues Roles
| Residue | Roles |
|---|---|
| Gln17(16)A | hydrogen bond donor, electrostatic stabiliser |
| Glu140(139)A (main-N) | hydrogen bond donor |
| Asp169(168)A | covalently attached |
| Gly260(259)B (main-C) | hydrogen bond acceptor |
| Gly261(260)B (main-N) | hydrogen bond donor |
Chemical Components
ingold: unimolecular elimination by the conjugate base, enzyme-substrate complex cleavage, intermediate collapse, intermediate formation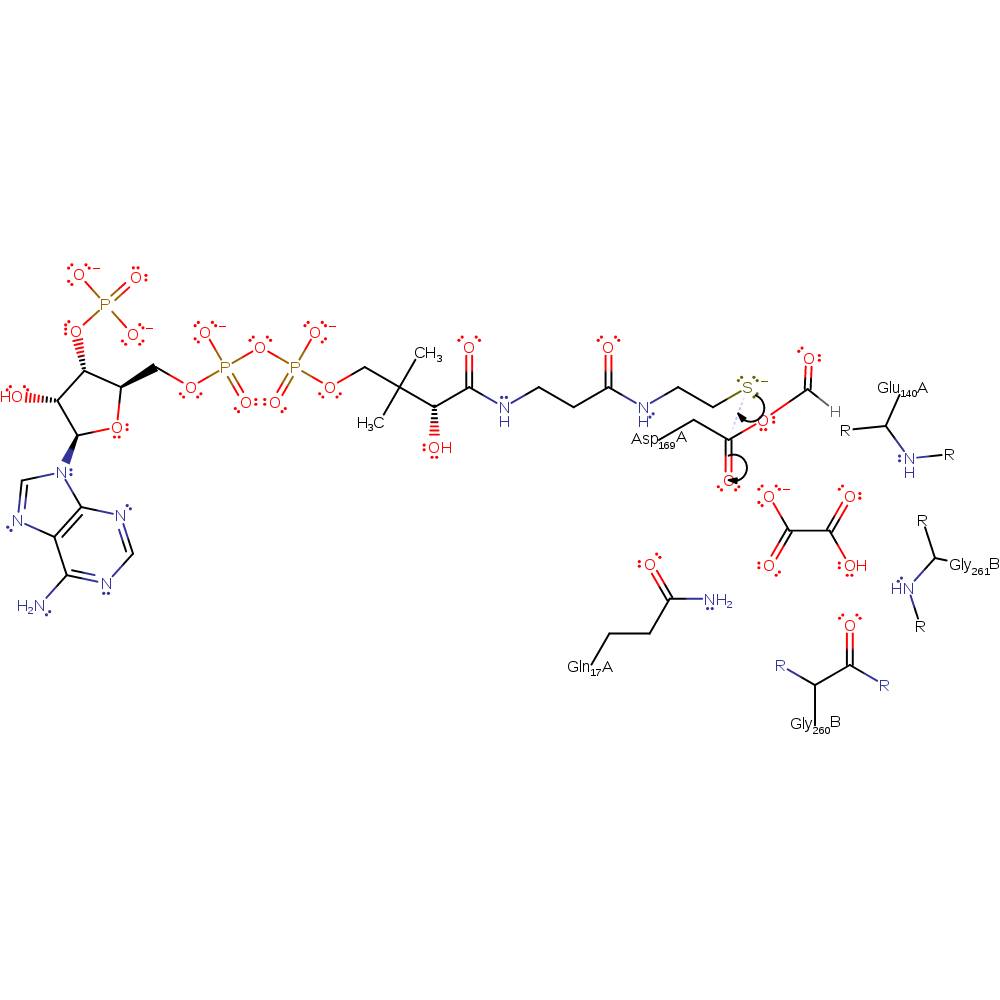
Step 3. CoAS performs a nucleophilic attack on the mixed anhydride, resulting in the beta-aspartyl-CoA thioester
Download: Image, Marvin FileCatalytic Residues Roles
| Residue | Roles |
|---|---|
| Glu140(139)A (main-N) | hydrogen bond donor |
| Gly261(260)B (main-N) | hydrogen bond donor |
| Gln17(16)A | hydrogen bond donor |
| Gly260(259)B (main-C) | hydrogen bond acceptor |
| Gln17(16)A | electrostatic stabiliser |
| Asp169(168)A | covalently attached, electrophile |
Chemical Components
ingold: bimolecular nucleophilic addition, enzyme-substrate complex formation, intermediate formation
Step 4. The oxyanion initiates an elimination of formate and leaving Asp169 still covalently attached to CoAS.
Download: Image, Marvin FileCatalytic Residues Roles
| Residue | Roles |
|---|---|
| Gln17(16)A | hydrogen bond donor |
| Glu140(139)A (main-N) | hydrogen bond donor |
| Gly261(260)B (main-N) | hydrogen bond donor |
| Gly260(259)B (main-C) | hydrogen bond acceptor |
| Asp169(168)A | covalently attached |
| Gln17(16)A | electrostatic stabiliser |
| Asp169(168)A | electrofuge |
Chemical Components
ingold: unimolecular elimination by the conjugate base, enzyme-substrate complex cleavage, intermediate collapse, intermediate formation, overall product formed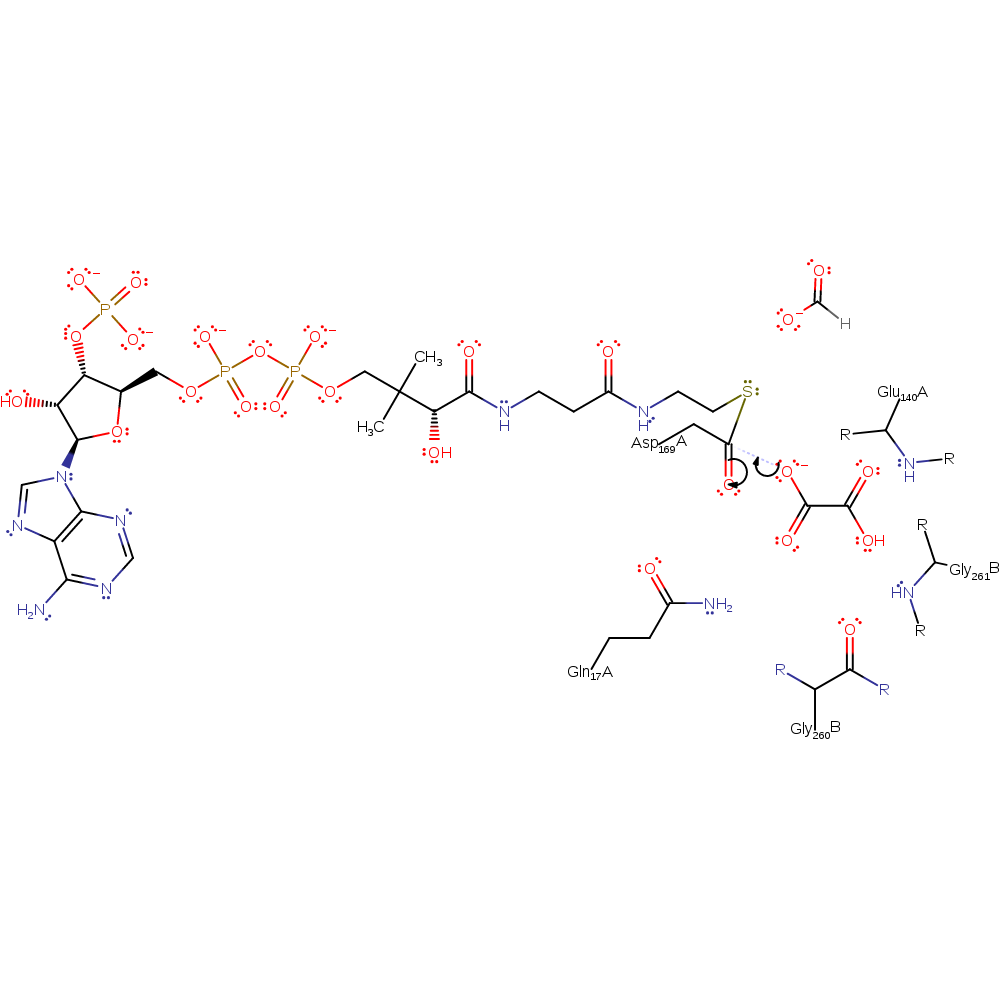
Step 5. The oxalate attacks the Asp169 carboxylate carbon in a nucleophilic addition.
Download: Image, Marvin FileCatalytic Residues Roles
| Residue | Roles |
|---|---|
| Gln17(16)A | hydrogen bond donor |
| Glu140(139)A (main-N) | hydrogen bond donor |
| Gly261(260)B (main-N) | hydrogen bond donor |
| Gly260(259)B (main-C) | hydrogen bond acceptor |
| Gln17(16)A | electrostatic stabiliser |
| Asp169(168)A | covalently attached |
Chemical Components
ingold: bimolecular nucleophilic addition, overall reactant used, enzyme-substrate complex formation, intermediate formationCatalytic Residues Roles
| Residue | Roles |
|---|---|
| Gln17(16)A | hydrogen bond donor |
| Glu140(139)A (main-N) | hydrogen bond donor |
| Gly261(260)B (main-N) | hydrogen bond donor |
| Gly260(259)B (main-C) | hydrogen bond acceptor |
| Gln17(16)A | electrostatic stabiliser |
| Asp169(168)A | covalently attached |
| Asp169(168)A | electrofuge |
Chemical Components
ingold: unimolecular elimination by the conjugate base, enzyme-substrate complex cleavage, intermediate collapse, intermediate formation
Step 7. CoAS attacks the carbonyl carbon of the bound oxalate in a nucleophilic addition.
Download: Image, Marvin FileCatalytic Residues Roles
| Residue | Roles |
|---|---|
| Gln17(16)A | hydrogen bond donor |
| Glu140(139)A (main-N) | hydrogen bond donor |
| Gly261(260)B (main-N) | hydrogen bond donor |
| Gly260(259)B (main-C) | hydrogen bond acceptor |
| Asp169(168)A | covalently attached |
| Gln17(16)A | electrostatic stabiliser |
Chemical Components
ingold: bimolecular nucleophilic addition, enzyme-substrate complex formation, intermediate formationCatalytic Residues Roles
| Residue | Roles |
|---|---|
| Gln17(16)A | hydrogen bond donor |
| Glu140(139)A (main-N) | hydrogen bond donor |
| Gly261(260)B (main-N) | hydrogen bond donor |
| Gly260(259)B (main-C) | hydrogen bond acceptor |
| Gln17(16)A | electrostatic stabiliser |
| Asp169(168)A | nucleofuge |
Chemical Components
ingold: unimolecular elimination by the conjugate base, enzyme-substrate complex cleavage, intermediate collapse, intermediate terminated, overall product formed, native state of enzyme regeneratedIntroduction
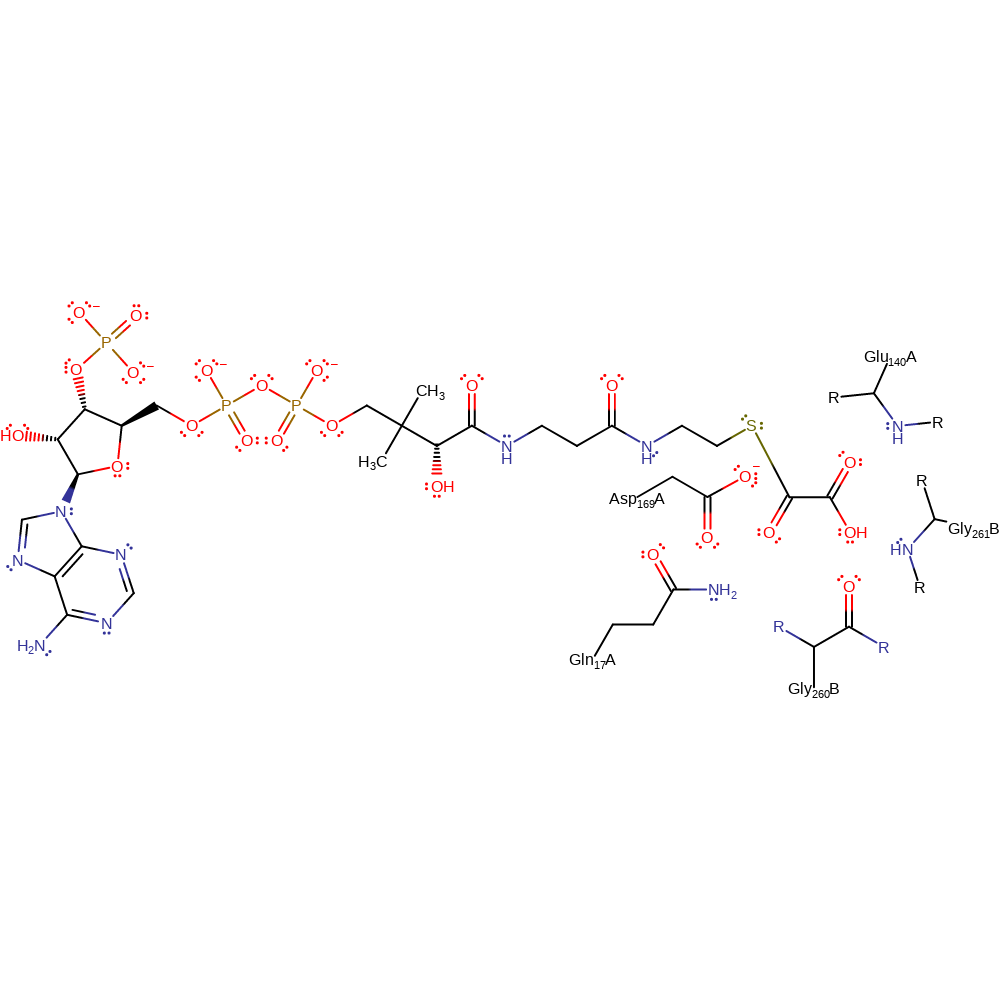
Asp 169 performs nucleophilic attack upon formyl-CoA, attacking the carbonyl group of the thioester. A tetrahedral oxyanion transition state is formed. It is unclear how the transition state is stabilised. The transition state collapses and the thiol-CoA group leaves. Next, oxalate performs a nucleophilic attack upon the electrophilic carbonyl group of Asp 169, within the anhydride intermediate. A tetrahedral oxyanion transition state is formed. The transition state collapses and the formate leaves. A second anhydride intermediate is formed. This is stabilised by hydrogen bonding of the oxalyl part of the oxalyl aspartic anhydride to stabilising residues. O1 is stabilised by hydrogen bonding to the backbone amide of Glu 140, O2 to the amide of Gly 261' and backbone carbonyl of Gly 260' and O3 to the backbone amide of Gln 17. The final stage of the reaction occurs when the thiol group of the CoA performs a nucleophilic attack upon the carbonyl group of oxalate, within the anhydride intermediate. A tetrahedral oxyanion transition state is formed. The transition state collapses and the Asp 169 residue leaves.
Catalytic Residues Roles
| UniProt | PDB* (1t4c) | ||
| Glu140 (main-N) | Glu140(139)A (main-N) | Glu 140 stabilises O1 of the oxalyl part of the oxalyl aspartic anhydride through hydrogen bonding to the backbone amide | hydrogen bond donor, electrostatic stabiliser |
| Gly260 (main-C) | Gly260(259)B (main-C) | Gly 260' stabilises O2 of the oxalyl part of the oxalyl aspartic anhydride through hydrogen bonding to the backbone carbonyl. | hydrogen bond acceptor, electrostatic stabiliser |
| Gly261 (main-N) | Gly261(260)B (main-N) | Gly 261' stabilises O2 of the oxalyl part of the oxalyl aspartic anhydride through hydrogen bonding to the backbone amide | hydrogen bond donor, electrostatic stabiliser |
| Gln17 | Gln17(16)A | Gln 17 stabilises O3 of the oxalyl part of the oxalyl aspartic anhydride through hydrogen bonding to the backbone amide. | hydrogen bond donor, electrostatic stabiliser |
| Asp169 | Asp169(168)A | Asp 169 performs nucleophilic attack upon formyl-CoA, attacking the carbonyl group of the thioester. The electrophilic carbonyl group of Asp 169 is nucleophilically attacked by oxalate. | covalently attached, nucleofuge, nucleophile, electrofuge, electrophile |
Chemical Components
bimolecular nucleophilic addition, overall reactant used, enzyme-substrate complex formation, intermediate formation, unimolecular elimination by the conjugate base, enzyme-substrate complex cleavage, intermediate collapse, overall product formed, intermediate terminated, native state of enzyme regeneratedReferences
- Jonsson S et al. (2004), J Biol Chem, 279, 36003-36012. Kinetic and Mechanistic Characterization of the Formyl-CoA Transferase from Oxalobacter formigenes. DOI:10.1074/jbc.m404873200. PMID:15213226.
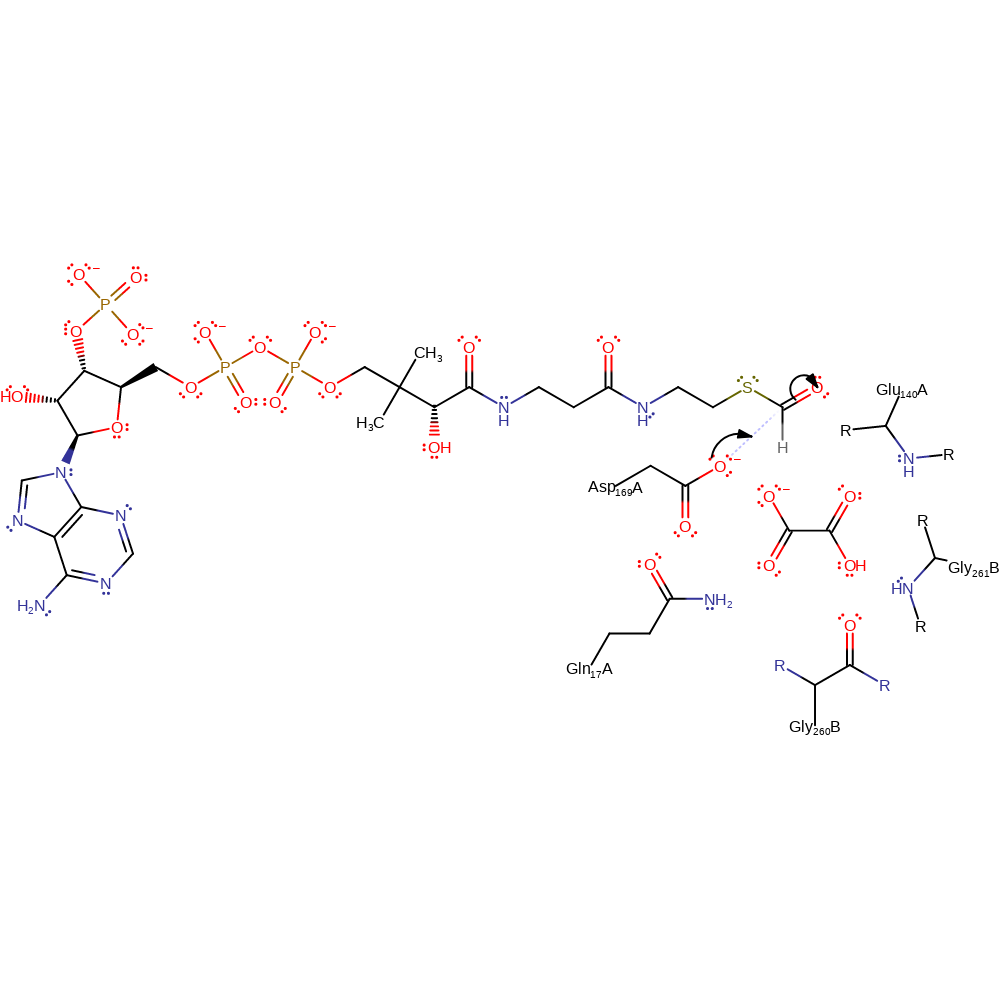
Step 1. Asp169 attacks the carbonyl carbon of formyl-CoA in a nucleophilic addition.
Download: Image, Marvin FileCatalytic Residues Roles
| Residue | Roles |
|---|---|
| Gln17(16)A | hydrogen bond donor, electrostatic stabiliser |
| Glu140(139)A (main-N) | hydrogen bond donor |
| Gly260(259)B (main-C) | hydrogen bond acceptor |
| Gly261(260)B (main-N) | hydrogen bond donor |
| Asp169(168)A | nucleophile |
Chemical Components
ingold: bimolecular nucleophilic addition, overall reactant used, enzyme-substrate complex formation, intermediate formation
Step 2. The oxyanion initiates an elimination of the CoA, leaving the Asp169 acylated.
Download: Image, Marvin FileCatalytic Residues Roles
| Residue | Roles |
|---|---|
| Gln17(16)A | hydrogen bond donor, electrostatic stabiliser |
| Glu140(139)A (main-N) | hydrogen bond donor |
| Asp169(168)A | covalently attached |
| Gly260(259)B (main-C) | hydrogen bond acceptor |
| Gly261(260)B (main-N) | hydrogen bond donor |
Chemical Components
ingold: unimolecular elimination by the conjugate base, enzyme-substrate complex cleavage, intermediate collapse, intermediate formation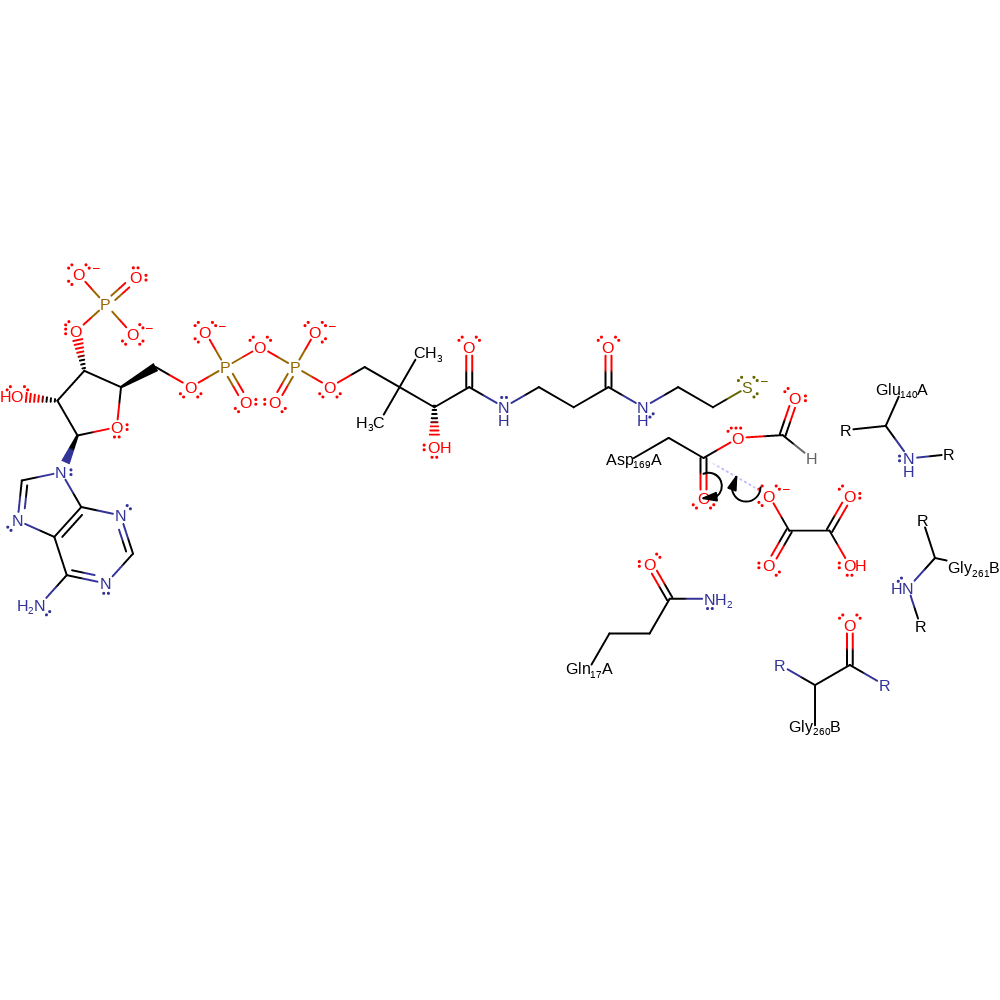
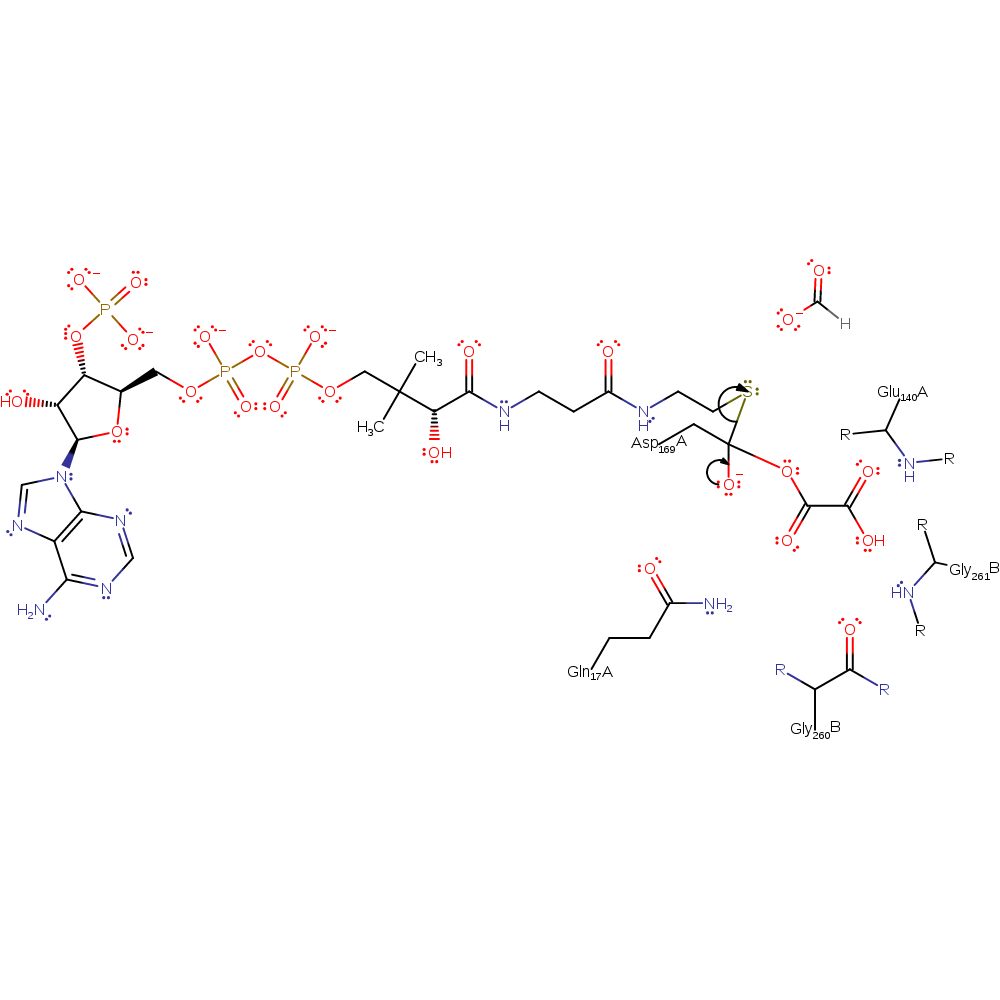
Step 3. The oxalate attacks the Asp169 carboxylate carbon in a nucleophilic addition.
Download: Image, Marvin FileCatalytic Residues Roles
| Residue | Roles |
|---|---|
| Gln17(16)A | hydrogen bond donor, electrostatic stabiliser |
| Glu140(139)A (main-N) | hydrogen bond donor |
| Asp169(168)A | covalently attached |
| Gly260(259)B (main-C) | hydrogen bond acceptor |
| Gly261(260)B (main-N) | hydrogen bond donor |
| Asp169(168)A | electrophile |
Chemical Components
ingold: bimolecular nucleophilic addition, overall reactant used, enzyme-substrate complex formation, intermediate formationCatalytic Residues Roles
| Residue | Roles |
|---|---|
| Gln17(16)A | hydrogen bond donor |
| Glu140(139)A (main-N) | hydrogen bond donor, electrostatic stabiliser |
| Asp169(168)A | covalently attached |
| Gly260(259)B (main-C) | hydrogen bond acceptor, electrostatic stabiliser |
| Gly261(260)B (main-N) | hydrogen bond donor, electrostatic stabiliser |
| Asp169(168)A | electrofuge |
Chemical Components
ingold: unimolecular elimination by the conjugate base, enzyme-substrate complex cleavage, intermediate collapse, intermediate formation, overall product formed
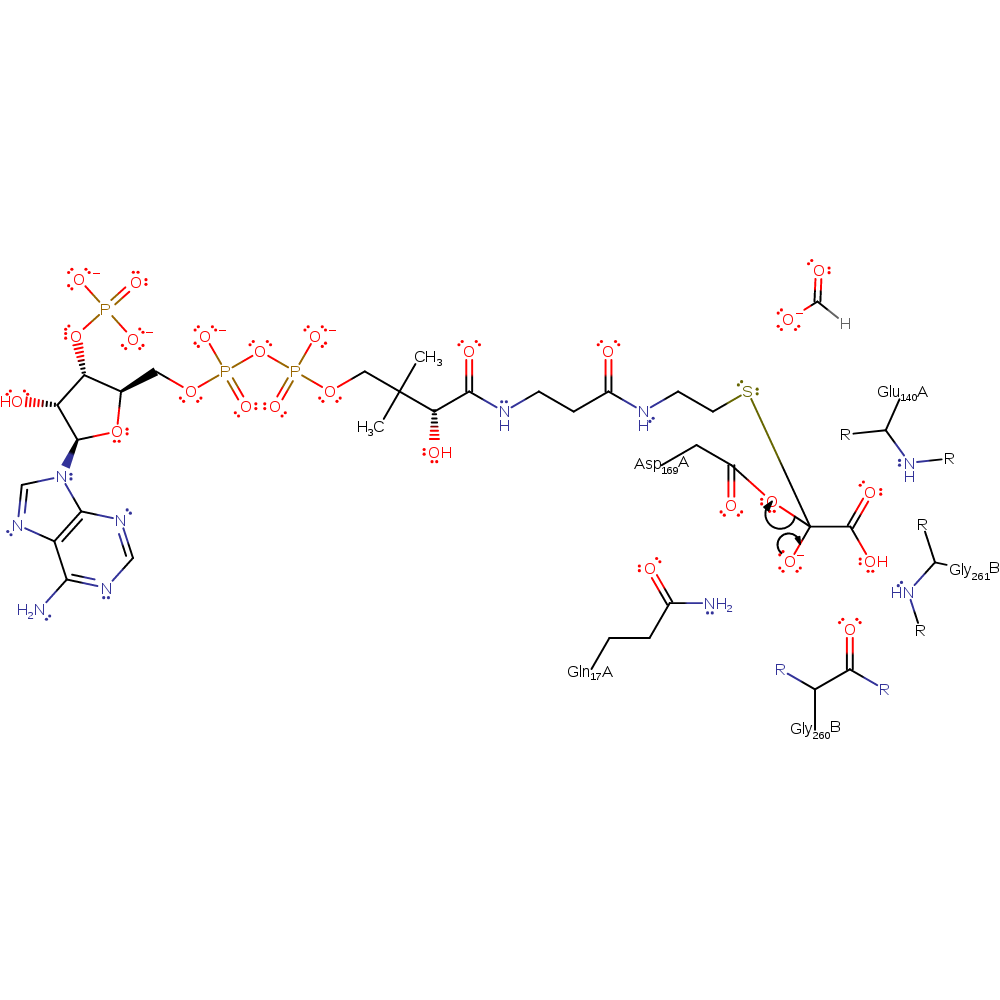
Step 5. The thiolate of CoA attacks the carbonyl carbon of the covalently attached oxalyl group in a nucleophilic addition.
Download: Image, Marvin FileCatalytic Residues Roles
| Residue | Roles |
|---|---|
| Gln17(16)A | hydrogen bond donor |
| Glu140(139)A (main-N) | hydrogen bond donor |
| Asp169(168)A | covalently attached |
| Gly260(259)B (main-C) | hydrogen bond acceptor |
| Gly261(260)B (main-N) | hydrogen bond donor |
Chemical Components
ingold: bimolecular nucleophilic addition, enzyme-substrate complex formation, intermediate formation
Step 6. The oxyanion initiates an elimination reaction that releases the product oxalyl-CoA and regenerated Asp69.
Download: Image, Marvin FileCatalytic Residues Roles
| Residue | Roles |
|---|---|
| Gln17(16)A | hydrogen bond donor, electrostatic stabiliser |
| Glu140(139)A (main-N) | hydrogen bond donor |
| Gly260(259)B (main-C) | hydrogen bond acceptor |
| Gly261(260)B (main-N) | hydrogen bond donor |
| Asp169(168)A | nucleofuge |




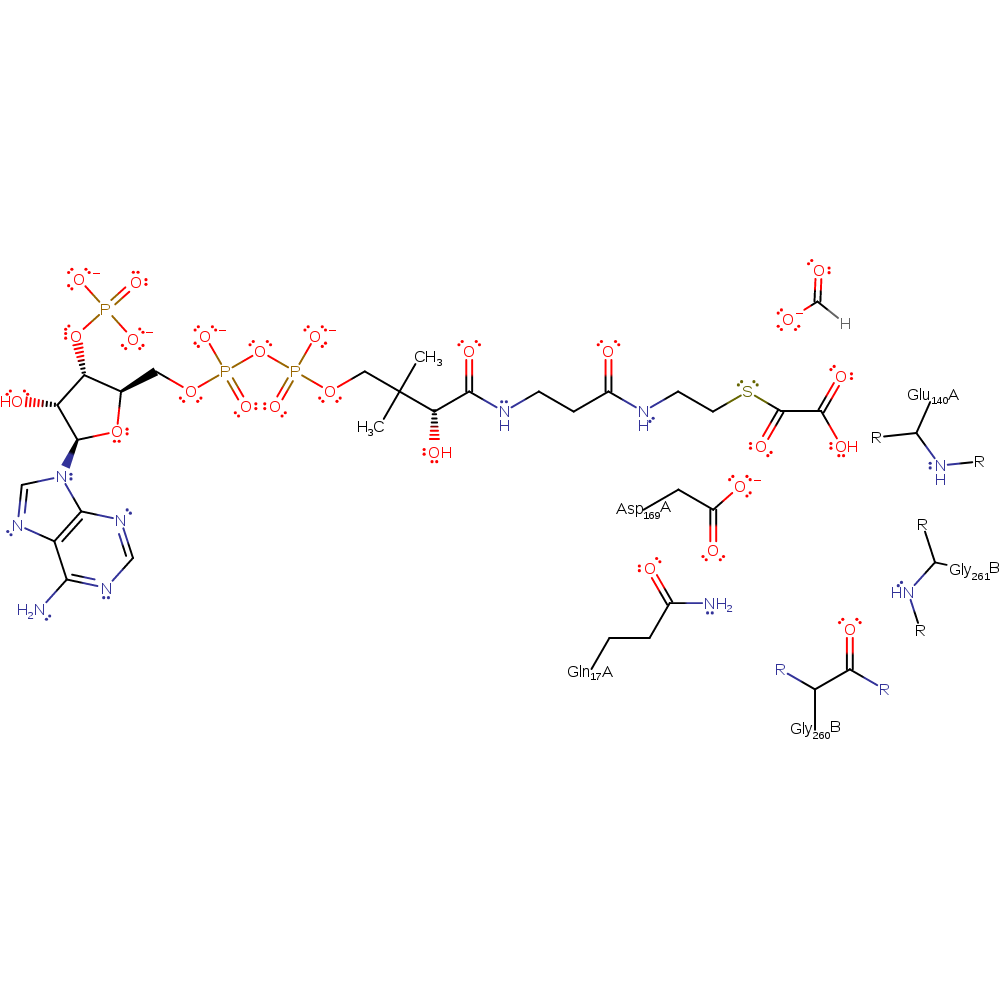 Download:
Download: 
 Download:
Download: