 |
PDBsum entry 5dxf
 |
|
|
 |
 |
 |
 |
Enzyme class:
|
 |
E.C.2.4.1.15
- alpha,alpha-trehalose-phosphate synthase (UDP-forming).
|
|
 |
 |
 |
 |
 |
Reaction:
|
 |
D-glucose 6-phosphate + UDP-alpha-D-glucose = alpha,alpha-trehalose 6-phosphate + UDP + H+
|
 |
 |
 |
 |
 |
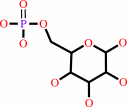 D-glucose 6-phosphate
D-glucose 6-phosphate
|
+
|
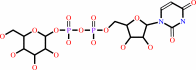 UDP-alpha-D-glucose
UDP-alpha-D-glucose
|
=
|
alpha,alpha-trehalose 6-phosphate
|
+
|
 UDP
UDP
|
+
|
H(+)
|
|
 |
 |
 |
 |
 |
 |
 |
 |
 |
 |
|
Molecule diagrams generated from .mol files obtained from the
KEGG ftp site
|
|
 |
 |
 |
 |
 |
 |
 |
 |
 |
 |
 |
 |
 |
 |
 |

|

|

|
| |
|
|
| |
|
DOI no:
|
Proc Natl Acad Sci U S A
113:7148-7153
(2016)
|
|
PubMed id:
|
|
|
|

|
| |
|
Structures of trehalose-6-phosphate phosphatase from pathogenic fungi reveal the mechanisms of substrate recognition and catalysis.
|
|
Y.Miao,
J.L.Tenor,
D.L.Toffaletti,
E.J.Washington,
J.Liu,
W.R.Shadrick,
M.A.Schumacher,
R.E.Lee,
J.R.Perfect,
R.G.Brennan.
|
|
|

|
| |
ABSTRACT
|
|

|
| |

|
Trehalose is a disaccharide essential for the survival and virulence of
pathogenic fungi. The biosynthesis of trehalose requires trehalose-6-phosphate
synthase, Tps1, and trehalose-6-phosphate phosphatase, Tps2. Here, we report the
structures of the N-terminal domain of Tps2 (Tps2NTD) from Candida albicans, a
transition-state complex of the Tps2 C-terminal trehalose-6-phosphate
phosphatase domain (Tps2PD) bound to BeF3 and trehalose, and catalytically dead
Tps2PD(D24N) from Cryptococcus neoformans bound to trehalose-6-phosphate (T6P).
The Tps2NTD closely resembles the structure of Tps1 but lacks any catalytic
activity. The Tps2PD-BeF3-trehalose and Tps2PD(D24N)-T6P complex structures
reveal a "closed" conformation that is effected by extensive
interactions between each trehalose hydroxyl group and residues of the cap and
core domains of the protein, thereby providing exquisite substrate specificity.
Disruption of any of the direct substrate-protein residue interactions leads to
significant or complete loss of phosphatase activity. Notably, the
Tps2PD-BeF3-trehalose complex structure captures an aspartyl-BeF3 covalent
adduct, which closely mimics the proposed aspartyl-phosphate intermediate of the
phosphatase catalytic cycle. Structures of substrate-free Tps2PD reveal an
"open" conformation whereby the cap and core domains separate and
visualize the striking conformational changes effected by substrate binding and
product release and the role of two hinge regions centered at approximately
residues 102-103 and 184-188. Significantly, tps2Δ, tps2NTDΔ, and tps2D705N
strains are unable to grow at elevated temperatures. Combined, these studies
provide a deeper understanding of the substrate recognition and catalytic
mechanism of Tps2 and provide a structural basis for the future design of novel
antifungal compounds against a target found in three major fungal pathogens.

|

|
|
|

|

|
|
 |
 |
|
 |
 |
 |
 |
 |
 |
 |
 |
 |
 |

