 |
PDBsum entry 5u05
|
|

|
|
PDB id:
|
 |
|
 |
| Name: |
 |
Ligase
|
 |
|
Title:
|
 |
Cryo-em structure of the e. Coli ctp synthase tetramer
|
|
Structure:
|
 |
Ctp synthase. Chain: a, b, c, d. Synonym: cytidine 5'-triphosphate synthase, cytidine triphosphate synthetase, ctps, utp--ammonia ligase. Engineered: yes
|
|
Source:
|
 |
Escherichia coli. Organism_taxid: 562. Gene: pyrg, ecs88_3048. Expressed in: escherichia coli. Expression_system_taxid: 562
|
|
Authors:
|
 |
E.M.Lynch,D.R.Hicks,J.M.Kollman
|
|
Key ref:
|
 |
E.M.Lynch
et al.
(2017).
Human CTP synthase filament structure reveals the active enzyme conformation.
Nat Struct Mol Biol,
24,
507-514.
PubMed id:
|
 |
|
Date:
|
 |
|
22-Nov-16
|
Release date:
|
26-Apr-17
|
|
|

|

|
|
PROCHECK
|

|

|
|
|
Headers
|
 |
|
|
References
|
|
|
|

|

|
|
P0A7E5
(PYRG_ECOLI) -
CTP synthase from Escherichia coli (strain K12)
|
|

|

|
Seq:
Struc:
|
 |
 |
 |
545 a.a.
534 a.a.
|

|

|

|

|
|

|

|

|
 |
 |

|
|
Key: |
 |
PfamA domain |
 |
 |
 |
Secondary structure |
 |
|

|
|
|
 |
|
|
 |
 |
 |
 |
Enzyme class:
|
 |
E.C.6.3.4.2
- Ctp synthase (glutamine hydrolyzing).
|
|
 |
 |
 |
 |
 |
Reaction:
|
 |
UTP + L-glutamine + ATP + H2O = CTP + L-glutamate + ADP + phosphate + 2 H+
|
 |
 |
 |
 |
 |
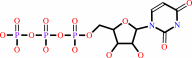 UTP
UTP
|
+
|
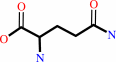 L-glutamine
L-glutamine
|
+
|
 ATP
ATP
|
+
|
H2O
|
=
|
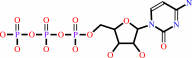 CTP
CTP
|
+
|
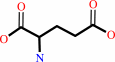 L-glutamate
L-glutamate
|
+
|
 ADP
ADP
|
+
|
 phosphate
phosphate
|
+
|
2
×
H(+)
|
|
 |
 |
 |
 |
 |
 |
 |
 |
 |
 |
|
Molecule diagrams generated from .mol files obtained from the
KEGG ftp site
|
|
 |
 |
 |
 |
 |
 |
 |
 |
 |
 |
 |
 |
 |
 |
 |

|

|

|
| |
|
|
| |
|
|
Nat Struct Mol Biol
24:507-514
(2017)
|
|
PubMed id:
|
|
|
|

|
| |
|
Human CTP synthase filament structure reveals the active enzyme conformation.
|
|
E.M.Lynch,
D.R.Hicks,
M.Shepherd,
J.A.Endrizzi,
A.Maker,
J.M.Hansen,
R.M.Barry,
Z.Gitai,
E.P.Baldwin,
J.M.Kollman.
|
|
|

|
| |
ABSTRACT
|
|

|
| |

|
The universally conserved enzyme CTP synthase (CTPS) forms filaments in bacteria
and eukaryotes. In bacteria, polymerization inhibits CTPS activity and is
required for nucleotide homeostasis. Here we show that for human CTPS,
polymerization increases catalytic activity. The cryo-EM structures of bacterial
and human CTPS filaments differ considerably in overall architecture and in the
conformation of the CTPS protomer, explaining the divergent consequences of
polymerization on activity. The structure of human CTPS filament, the first
structure of the full-length human enzyme, reveals a novel active conformation.
The filament structures elucidate allosteric mechanisms of assembly and
regulation that rely on a conserved conformational equilibrium. The findings may
provide a mechanism for increasing human CTPS activity in response to metabolic
state and challenge the assumption that metabolic filaments are generally
storage forms of inactive enzymes. Allosteric regulation of CTPS polymerization
by ligands likely represents a fundamental mechanism underlying assembly of
other metabolic filaments.

|

|
|
|

|

|
|
 |
 |
|
 |
 |
 |
 |
 |
 |
 |
 |
 |
 |

