 |
PDBsum entry 4z64
|
|
 |
|
|
 |
 |
 |
 |
Enzyme class 2:
|
 |
Chains A, C:
E.C.2.7.11.1
- non-specific serine/threonine protein kinase.
|
|
 |
 |
 |
 |
 |
Reaction:
|
 |
|
1.
|
L-seryl-[protein] + ATP = O-phospho-L-seryl-[protein] + ADP + H+
|
|
2.
|
L-threonyl-[protein] + ATP = O-phospho-L-threonyl-[protein] + ADP + H+
|
|
 |
 |
 |
 |
 |
L-seryl-[protein]
|
+
|
ATP
Bound ligand (Het Group name = )
matches with 48.00% similarity
|
=
|
O-phospho-L-seryl-[protein]
|
+
|
 ADP
ADP
|
+
|
H(+)
|
|
 |
 |
 |
 |
 |
L-threonyl-[protein]
|
+
|
 ATP
ATP
|
=
|
O-phospho-L-threonyl-[protein]
|
+
|
 ADP
ADP
|
+
|
H(+)
|
|
 |
 |
 |
 |
 |
 |
 |
 |
Enzyme class 3:
|
 |
Chain A:
E.C.4.6.1.2
- guanylate cyclase.
|
|
 |
 |
 |
 |
 |
Reaction:
|
 |
GTP = 3',5'-cyclic GMP + diphosphate
|
 |
 |
 |
 |
 |
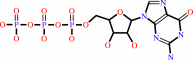 GTP
GTP
|
=
|
 3',5'-cyclic GMP
3',5'-cyclic GMP
|
+
|
diphosphate
Bound ligand (Het Group name = )
matches with 41.38% similarity
|
|
 |
 |
 |
 |
 |
 |
 |
 |
Enzyme class 4:
|
 |
Chain C:
E.C.2.7.10.1
- receptor protein-tyrosine kinase.
|
|
 |
 |
 |
 |
 |
Reaction:
|
 |
L-tyrosyl-[protein] + ATP = O-phospho-L-tyrosyl-[protein] + ADP + H+
|
 |
 |
 |
 |
 |
L-tyrosyl-[protein]
|
+
|
 ATP
ATP
|
=
|
O-phospho-L-tyrosyl-[protein]
|
+
|
 ADP
ADP
|
+
|
H(+)
|
|
 |
 |
 |
 |
 |
 |
 |
 |
 |
 |
|
Note, where more than one E.C. class is given (as above), each may
correspond to a different protein domain or, in the case of polyprotein
precursors, to a different mature protein.
|
|
 |
|
Molecule diagrams generated from .mol files obtained from the
KEGG ftp site
|
|
 |
 |
 |
 |
 |
 |
 |
 |
 |
 |
 |
 |
 |
 |
 |

|

|

|
| |
|
|
| |
|
DOI no:
|
Nature
525:265-268
(2015)
|
|
PubMed id:
|
|
|
|

|
| |
|
Allosteric receptor activation by the plant peptide hormone phytosulfokine.
|
|
J.Wang,
H.Li,
Z.Han,
H.Zhang,
T.Wang,
G.Lin,
J.Chang,
W.Yang,
J.Chai.
|
|
|

|
| |
ABSTRACT
|
|

|
| |

|
Phytosulfokine (PSK) is a disulfated pentapeptide that has a ubiquitous role in
plant growth and development. PSK is perceived by its receptor PSKR, a
leucine-rich repeat receptor kinase (LRR-RK). The mechanisms underlying the
recognition of PSK, the activation of PSKR and the identity of the components
downstream of the initial binding remain elusive. Here we report the crystal
structures of the extracellular LRR domain of PSKR in free, PSK- and
co-receptor-bound forms. The structures reveal that PSK interacts mainly with a
β-strand from the island domain of PSKR, forming an anti-β-sheet. The two
sulfate moieties of PSK interact directly with PSKR, sensitizing PSKR
recognition of PSK. Supported by biochemical, structural and genetic evidence,
PSK binding enhances PSKR heterodimerization with the somatic embryogenesis
receptor-like kinases (SERKs). However, PSK is not directly involved in
PSKR-SERK interaction but stabilizes PSKR island domain for recruitment of a
SERK. Our data reveal the structural basis for PSKR recognition of PSK and
allosteric activation of PSKR by PSK, opening up new avenues for the design of
PSKR-specific small molecules.

|

|
|
|

|

|
|
 |
 |
|
 |
 |
 |
 |
 |
 |
 |
 |
 |
 |
|

