 |
PDBsum entry 4lru
 |
|
|
 |
 |
 |
 |
Enzyme class:
|
 |
E.C.4.2.1.130
- D-lactate dehydratase.
|
|
 |
 |
 |
 |
 |
Reaction:
|
 |
methylglyoxal + H2O = (R)-lactate + H+
|
 |
 |
 |
 |
 |
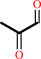 methylglyoxal
methylglyoxal
|
+
|
H2O
Bound ligand (Het Group name = )
matches with 80.00% similarity
|
=
|
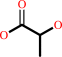 (R)-lactate
(R)-lactate
|
+
|
H(+)
|
|
 |
 |
 |
 |
 |
 |
 |
 |
 |
 |
|
Molecule diagrams generated from .mol files obtained from the
KEGG ftp site
|
|
 |
 |
 |
 |
 |
 |
 |
 |
 |
 |
 |
 |
 |
 |
 |

|

|

|
| |
|
|
| |
|
DOI no:
|
J Biol Chem
289:1662-1674
(2014)
|
|
PubMed id:
|
|
|
|

|
| |
|
A glutathione-independent glyoxalase of the DJ-1 superfamily plays an important role in managing metabolically generated methylglyoxal in Candida albicans.
|
|
S.Hasim,
N.A.Hussin,
F.Alomar,
K.R.Bidasee,
K.W.Nickerson,
M.A.Wilson.
|
|
|

|
| |
ABSTRACT
|
|

|
| |

|
Methylglyoxal is a cytotoxic reactive carbonyl compound produced by central
metabolism. Dedicated glyoxalases convert methylglyoxal to d-lactate using
multiple catalytic strategies. In this study, the DJ-1 superfamily member ORF
19.251/GLX3 from Candida albicans is shown to possess glyoxalase activity,
making this the first demonstrated glutathione-independent glyoxalase in fungi.
The crystal structure of Glx3p indicates that the protein is a monomer
containing the catalytic triad Cys(136)-His(137)-Glu(168). Purified Glx3p has an
in vitro methylglyoxalase activity (Km = 5.5 mm and kcat = 7.8 s(-1)) that is
significantly greater than that of more distantly related members of the DJ-1
superfamily. A close Glx3p homolog from Saccharomyces cerevisiae (YDR533C/Hsp31)
also has glyoxalase activity, suggesting that fungal members of the Hsp31 clade
of the DJ-1 superfamily are all probable glutathione-independent glyoxalases. A
homozygous glx3 null mutant in C. albicans strain SC5314 displays greater
sensitivity to millimolar levels of exogenous methylglyoxal, elevated levels of
intracellular methylglyoxal, and carbon source-dependent growth defects,
especially when grown on glycerol. These phenotypic defects are complemented by
restoration of the wild-type GLX3 locus. The growth defect of Glx3-deficient
cells in glycerol is also partially complemented by added inorganic phosphate,
which is not observed for wild-type or glucose-grown cells. Therefore, C.
albicans Glx3 and its fungal homologs are physiologically relevant
glutathione-independent glyoxalases that are not redundant with the previously
characterized glutathione-dependent GLO1/GLO2 system. In addition to its role in
detoxifying glyoxals, Glx3 and its close homologs may have other important roles
in stress response.

|

|
|
|

|

|
|
 |
 |
|
 |
 |
 |
 |
 |
 |
 |
 |
 |
 |

