 |
PDBsum entry 2b76

|
|
|

|
 |

|

|

|

|

|

|

|

|

|

|

|

|

|

|

|

|
 |

|

|

|
 |
 |
 |
 |
 |
 |
 |
 |
 |
 |
 |
 |
 |
 |
 |
 |
 |
 |
 |

|

|

|

|

|

|

|

|

|
|
Oxidoreductase
|
PDB id
|
|

|

|
2b76
|

|

|

|

|

|

|

|

|

|

|

|

|

|

|

|

|

|

|

|
|
 |
Contents |
 |
|
|

|

|

|

|

|

|

|

 577 a.a.
577 a.a.
|
 |

|

|

|

|

|

|

|

 243 a.a.
243 a.a.
|
 |

|

|

|

|

|

|

|

 130 a.a.
130 a.a.
|
 |

|

|

|

|

|

|

|

 119 a.a.
119 a.a.
|
 |

|

|
|
|
|
|
* Residue conservation analysis
|
|
|

|
|
PDB id:
|
 |
|
 |
| Name: |
 |
Oxidoreductase
|
 |
|
Title:
|
 |
E. Coli quinol fumarate reductase frda e49q mutation
|
|
Structure:
|
 |
Fumarate reductase flavoprotein subunit. Chain: a, m. Engineered: yes. Mutation: yes. Fumarate reductase iron-sulfur protein. Chain: b, n. Engineered: yes. Fumarate reductase subunit c. Chain: c, o.
|
|
Source:
|
 |
Escherichia coli. Organism_taxid: 562. Gene: frda. Expressed in: escherichia coli. Expression_system_taxid: 562. Gene: frdb. Gene: frdc. Gene: frdd.
|
|
Biol. unit:
|
 |
 Tetramer (from
)
Tetramer (from
)
|
|
Resolution:
|
 |
|
3.30Å
|
R-factor:
|
0.248
|
R-free:
|
0.284
|
|
|
Authors:
|
 |
E.Maklashina,T.M.Iverson,Y.Sher,V.Kotlyar,O.Mirza,J.Andrell, J.M.Hudson,F.A.Armstrong,G.Cecchini
|
Key ref:

|
 |
E.Maklashina
et al.
(2006).
Fumarate reductase and succinate oxidase activity of Escherichia coli complex II homologs are perturbed differently by mutation of the flavin binding domain.
J Biol Chem,
281,
11357-11365.
PubMed id:
DOI:

|
 |
|
Date:
|
 |
|
03-Oct-05
|
Release date:
|
21-Feb-06
|
|
|

|

|
|
PROCHECK
|

|

|
|
|
Headers
|
 |
|
|
References
|
|
|
|

|

|
|
P00363
(FRDA_ECOLI) -
Fumarate reductase flavoprotein subunit from Escherichia coli (strain K12)
|
|

|

|
Seq:
Struc:
|
 |
 |
 |
602 a.a.
577 a.a.*
|

|

|

|

|
|

|

|

|
 |
 |

|

|

|
|
P0AC47
(FRDB_ECOLI) -
Fumarate reductase iron-sulfur subunit from Escherichia coli (strain K12)
|
|

|

|
Seq:
Struc:
|
 |
 |
 |
244 a.a.
243 a.a.
|

|

|

|

|
|

|

|

|
 |
 |

|
 |
|
|
 |
 |
 |
 |
Enzyme class:
|
 |
Chains A, B, M, N:
E.C.1.3.5.1
- succinate dehydrogenase.
|
|
 |
 |
 |
 |
 |

Pathway:
|
 |
Citric acid cycle
|
 |
 |
 |
 |
 |
Reaction:
|
 |
a quinone + succinate = fumarate + a quinol
|
 |
 |
 |
 |
 |
quinone
Bound ligand (Het Group name = )
matches with 61.54% similarity
|
+
|
 succinate
succinate
|
=
|
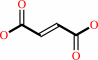 fumarate
fumarate
|
+
|
quinol
|
|
 |
 |
 |
 |
 |
 |
 |
 |
Cofactor:
|
 |
FAD; Iron-sulfur
|
 |
 |
 |
 |
 |
 FAD
FAD
|
 Iron-sulfur
Iron-sulfur
|
|
 |
 |
 |
 |
|
Molecule diagrams generated from .mol files obtained from the
KEGG ftp site
|
|
 |
 |
 |
 |
 |
 |
 |
 |
 |
 |
 |
 |
 |
 |
 |

|

|

|
| |
|
|
| |
|
DOI no:
|
J Biol Chem
281:11357-11365
(2006)
|
|
PubMed id:
|
|
|
|

|
| |
|
Fumarate reductase and succinate oxidase activity of Escherichia coli complex II homologs are perturbed differently by mutation of the flavin binding domain.
|
|
E.Maklashina,
T.M.Iverson,
Y.Sher,
V.Kotlyar,
J.Andréll,
O.Mirza,
J.M.Hudson,
F.A.Armstrong,
R.A.Rothery,
J.H.Weiner,
G.Cecchini.
|
|
|

|
| |
ABSTRACT
|
|

|
| |

|
The Escherichia coli complex II homologues succinate:ubiquinone oxidoreductase
(SQR, SdhCDAB) and menaquinol:fumarate oxidoreductase (QFR, FrdABCD) have
remarkable structural homology at their dicarboxylate binding sites. Although
both SQR and QFR can catalyze the interconversion of fumarate and succinate, QFR
is a much better fumarate reductase, and SQR is a better succinate oxidase. An
exception to the conservation of amino acids near the dicarboxylate binding
sites of the two enzymes is that there is a Glu (FrdA Glu-49) near the
covalently bound FAD cofactor in most QFRs, which is replaced with a Gln (SdhA
Gln-50) in SQRs. The role of the amino acid side chain in enzymes with
Glu/Gln/Ala substitutions at FrdA Glu-49 and SdhA Gln-50 has been investigated
in this study. The data demonstrate that the mutant enzymes with Ala
substitutions in either QFR or SQR remain functionally similar to their wild
type counterparts. There were, however, dramatic changes in the catalytic
properties when Glu and Gln were exchanged for each other in QFR and SQR. The
data show that QFR and SQR enzymes are more efficient succinate oxidases when
Gln is in the target position and a better fumarate reductase when Glu is
present. Overall, structural and catalytic analyses of the FrdA E49Q and SdhA
Q50E mutants suggest that coulombic effects and the electronic state of the FAD
are critical in dictating the preferred directionality of the succinate/fumarate
interconversions catalyzed by the complex II superfamily.

|

|
|
|

|
| |
Selected figure(s)
|
|

|
| |
 |
 |

|
 |

|
 |
Figure 2.
FIGURE 2. Menaquinol-fumarate reductase reaction catalyzed
by QFR and FrdA E49Q. The reaction was assayed as described
under "Materials and Methods" under anaerobic conditions in the
presence of DT diaphorase and NADH to continuously regenerate
MQ[1]H[2]. A, fumarate dependence of the rate of the
MQ[1]H[2]-fumarate reductase reaction. B, reduction of 15
µM fumarate by wild type QFR and FrdA E49Q by MQ[1]H[2]
regenerated in the presence of NADH and DT diaphorase. WT, wild
type.
|
 |
Figure 7.
FIGURE 7. Comparison of the active site structures in wild
type (Protein Data Bank code 1KF6 [PDB]
) and FrdA E49Q QFR (Protein Data Bank code 2B76). The wild type
(teal) and FrdA E49Q variant (yellow) are superimposed in the
same view as Fig. 1. Only the main chains from the E49Q variant
are shown for clarity. Oxygen atoms are in red, nitrogen atoms
are in blue, and carbon atoms follow the color of the molecule.
Little structural perturbation is observed, with a slight
movement of the side chain functional group of Arg-287. However,
an altered hydrogen-bonding pattern is observed, with a new
hydrogen bonding interaction to the side chain of Glu-245,
altered directionality of the hydrogen bond to Arg-287, and a
putative hydrogen-bonding interaction with the N-5 of the FAD.
The latter two have been shown to directly participate in the
fumarate reduction reaction.
|
 |
|
|

|
| |
The above figures are
reprinted
by permission from the ASBMB:
J Biol Chem
(2006,
281,
11357-11365)
copyright 2006.
|
|
| |
Figures were
selected
by an automated process.
|
|

|

|
|
 |
 |
|
 |
 |
 |
 |
 |
 |
 |
 |
 |
|
Literature references that cite this PDB file's key reference
|
|
 |
| |
PubMed id
|
 |
Reference
|
 |
|
|
|
 |
J.Ruprecht,
V.Yankovskaya,
E.Maklashina,
S.Iwata,
and
G.Cecchini
(2009).
Structure of Escherichia coli succinate:quinone oxidoreductase with an occupied and empty quinone-binding site.
|
| |
J Biol Chem,
284,
29836-29846.
|
 |
|
PDB codes:
|
 |
|
|
|
|
|
 |
S.Al Dahouk,
S.Loisel-Meyer,
H.C.Scholz,
H.Tomaso,
M.Kersten,
A.Harder,
H.Neubauer,
S.Köhler,
and
V.Jubier-Maurin
(2009).
Proteomic analysis of Brucella suis under oxygen deficiency reveals flexibility in adaptive expression of various pathways.
|
| |
Proteomics,
9,
3011-3021.
|
 |
|
|
|
|
 |
T.M.Tomasiak,
E.Maklashina,
G.Cecchini,
and
T.M.Iverson
(2008).
A threonine on the active site loop controls transition state formation in Escherichia coli respiratory complex II.
|
| |
J Biol Chem,
283,
15460-15468.
|
 |
|
PDB code:
|
 |
|
|
|
|
|
 |
J.Borloo,
B.Vergauwen,
L.De Smet,
A.Brigé,
B.Motte,
B.Devreese,
and
J.Van Beeumen
(2007).
A kinetic approach to the dependence of dissimilatory metal reduction by Shewanella oneidensis MR-1 on the outer membrane cytochromes c OmcA and OmcB.
|
| |
FEBS J,
274,
3728-3738.
|
 |
|
 |
 |
|
The most recent references are shown first.
Citation data come partly from CiteXplore and partly
from an automated harvesting procedure. Note that this is likely to be
only a partial list as not all journals are covered by
either method. However, we are continually building up the citation data
so more and more references will be included with time.
Where a reference describes a PDB structure, the PDB
codes are
shown on the right.
|

| | |

