
|

|

|
|
 |
Contents |
 |
|
|

|

|

|

|

|

|

|
 588 a.a.
588 a.a.
|
 |

|

|

|

|

|

|

|
 238 a.a.
238 a.a.
|
 |

|

|

|

|

|

|

|
 129 a.a.
129 a.a.
|
 |

|

|

|

|

|

|

|
 113 a.a.
113 a.a.
|
 |

|

|
|
|
|
|
|
|
|
|
|

|
 |
Theoretical model |
 |
|
PDB id:
|
 |
|
 |
| Name: |
 |
Oxidoreductase/electron transport
|
 |
|
Title:
|
 |
Complex ii (succinate dehydrogenase) from e. Coli with carboxin inhibitor docked at the ubiquinone binding site
|
|
Structure:
|
 |
Succinate dehydrogenase flavoprotein subunit. Chain: a. Succinate dehydrogenase iron-sulfur protein. Chain: b. Succinate dehydrogenase cytochrome b556 subunit. Chain: c. Synonym: cytochrome b-556. Succinate dehydrogenase hydrophobic membrane anchor protein.
|
|
Source:
|
 |
Escherichia coli. Bacteria. Bacteria
|
|
Authors:
|
 |
R.Horsefield,V.Yankovskaya,G.Sexton,W.Whittingham,K.Shiomi, S.Omura,B.Byrne,G.Cecchini,S.Iwata
|
Key ref:

|
 |
R.Horsefield
et al.
(2006).
Structural and computational analysis of the quinone-binding site of complex II (succinate-ubiquinone oxidoreductase): a mechanism of electron transfer and proton conduction during ubiquinone reduction.
J Biol Chem,
281,
7309-7316.
PubMed id:
DOI:

|
 |
|
Date:
|
 |
|
19-Jul-05
|
Release date:
|
03-Jan-06
|
|
|

|

|
|
PROCHECK
|

|

|
|
|
Headers
|
 |
|
|
References
|
|

|
|
|
|
|

|

|
|

|

|
|
|

|

|

|
 |
 |

|

|

|
|
P07014
(SDHB_ECOLI) -
Succinate dehydrogenase iron-sulfur subunit from Escherichia coli (strain K12)
|
|

|

|
Seq:
Struc:
|
 |
 |
 |
238 a.a.
238 a.a.
|

|

|

|

|
|

|

|

|
 |
 |

|
 |
|
|
 |
 |
 |
 |
Enzyme class:
|
 |
Chains A, B:
E.C.1.3.99.1
- Deleted entry.
|
|
 |
 |
 |
 |
 |
Reaction:
|
 |
Succinate + acceptor = fumarate + reduced acceptor
|
 |
 |
 |
 |
 |
Succinate
Bound ligand (Het Group name = )
matches with 8889.00% similarity
corresponds exactly
+
|
acceptor
|
=
|
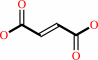 fumarate
fumarate
|
+
|
reduced acceptor
|
|  |
 |
 |
 |
 |
 |
 |
 |
Cofactor:
|
 |
FAD
|
 |
 |
 |
 |
 |
FAD
Bound ligand (Het Group name =
FAD)
matches with 41.33% similarity
|
|
 |
 |
 |
 |
|
Molecule diagrams generated from .mol files obtained from the
KEGG ftp site
|
|
 |
 |
 |
 |
 |
 |
 |
 |
 |
 |
 |
 |
 |
 |
 |

|

|

|
| |
|
|
| |
|
DOI no:
|
J Biol Chem
281:7309-7316
(2006)
|
|
PubMed id:
|
|
|
|

|
| |
|
Structural and computational analysis of the quinone-binding site of complex II (succinate-ubiquinone oxidoreductase): a mechanism of electron transfer and proton conduction during ubiquinone reduction.
|
|
R.Horsefield,
V.Yankovskaya,
G.Sexton,
W.Whittingham,
K.Shiomi,
S.Omura,
B.Byrne,
G.Cecchini,
S.Iwata.
|
|
|

|
| |
ABSTRACT
|
|

|
| |

|
The transfer of electrons and protons between membrane-bound respiratory
complexes is facilitated by lipid-soluble redox-active quinone molecules (Q).
This work presents a structural analysis of the quinone-binding site (Q-site)
identified in succinate:ubiquinone oxidoreductase (SQR) from Escherichia coli.
SQR, often referred to as Complex II or succinate dehydrogenase, is a functional
member of the Krebs cycle and the aerobic respiratory chain and couples the
oxidation of succinate to fumarate with the reduction of quinone to quinol
(QH(2)). The interaction between ubiquinone and the Q-site of the protein
appears to be mediated solely by hydrogen bonding between the O1 carbonyl group
of the quinone and the side chain of a conserved tyrosine residue. In this work,
SQR was co-crystallized with the ubiquinone binding-site inhibitor Atpenin A5
(AA5) to confirm the binding position of the inhibitor and reveal additional
structural details of the Q-site. The electron density for AA5 was located
within the same hydrophobic pocket as ubiquinone at, however, a different
position within the pocket. AA5 was bound deeper into the site prompting further
assessment using protein-ligand docking experiments in silico. The initial
interpretation of the Q-site was re-evaluated in the light of the new SQR-AA5
structure and protein-ligand docking data. Two binding positions, the Q(1)-site
and Q(2)-site, are proposed for the E. coli SQR quinone-binding site to explain
these data. At the Q(2)-site, the side chains of a serine and histidine residue
are suitably positioned to provide hydrogen bonding partners to the O4 carbonyl
and methoxy groups of ubiquinone, respectively. This allows us to propose a
mechanism for the reduction of ubiquinone during the catalytic turnover of the
enzyme.

|

|
|
|

|
| |
Selected figure(s)
|
|

|
| |
 |
 |

|
 |

|
 |
Figure 1.
FIGURE 1. Q-site in E. coli SQR; slab view with the surface
and interior of the protein shown in beige and gray,
respectively. Binding positions at the Q[1]-site for ubiquinone
in the native structure (blue, PDB 1NEK [PDB]
) (A) and DNP17 inhibitor (orange, PDB 1NEN) (B) and the
Q[2]-site for GOLD docked ubiquinone (green) (C) and carboxin
inhibitor (Cbx, purple, PDB 2AD0) (D). Key residues (gray) are
labeled, and protein-ligand interactions shown by red dotted
lines with the distances labeled.
|
 |
Figure 4.
FIGURE 4. Putative proton transfer pathway in E. coli SQR.
A, surface representation of the trimer with the opening of the
pathway to the cytoplasm viewed parallel to the membrane and B,
slab view from the cytoplasm along the membrane normal showing
the path of the chain from one side of the anchor monomer to the
Q-site on the other. The surface of the protein (beige), water
molecules (red space-fill), and ubiquinone (blue space-fill) are
shown. C, detailed view of the pathway from the cytoplasm (red)
to the Q-site (blue/green) in E. coli SQR; water molecules (red
space-fill) and conserved residue side chains (gray) interacting
with the pathway are shown. Ubiquinone (Q[1]-site, blue) and AA5
(Q[2]-site, green) at the Q-site are labeled accordingly.
Hydrogen bonds and other distances are shown by red and gray
dotted lines, respectively, and the distances are labeled.
|
 |
|
|

|
| |
The above figures are
reprinted
by permission from the ASBMB:
J Biol Chem
(2006,
281,
7309-7316)
copyright 2006.
|
|
| |
Figures were
selected
by the author.
|
|

|

|
|
 |
 |
|
 |
 |
 |
 |
 |
 |
 |
 |
 |
|
Literature references that cite this PDB file's key reference
|
|
 |
| |
PubMed id
|
 |
Reference
|
 |
|
|
|
 |
H.Ishii,
T.Miyamoto,
S.Ushio,
and
M.Kakishima
(2011).
Lack of cross-resistance to a novel succinate dehydrogenase inhibitor, fluopyram, in highly boscalid-resistant isolates of Corynespora cassiicola and Podosphaera xanthii.
|
| |
Pest Manag Sci,
67,
474-482.
|
 |
|
|
|
|
 |
Y.Shima,
Y.Ito,
H.Hatabayashi,
A.Koma,
and
K.Yabe
(2011).
Five carboxin-resistant mutants exhibited various responses to carboxin and related fungicides.
|
| |
Biosci Biotechnol Biochem,
75,
181-184.
|
 |
|
|
|
|
 |
G.Lenaz,
and
M.L.Genova
(2010).
Structure and organization of mitochondrial respiratory complexes: a new understanding of an old subject.
|
| |
Antioxid Redox Signal,
12,
961.
|
 |
|
|
|
|
 |
S.Grimaldi,
R.Arias-Cartin,
P.Lanciano,
S.Lyubenova,
B.Endeward,
T.F.Prisner,
A.Magalon,
and
B.Guigliarelli
(2010).
Direct evidence for nitrogen ligation to the high stability semiquinone intermediate in Escherichia coli nitrate reductase A.
|
| |
J Biol Chem,
285,
179-187.
|
 |
|
|
|
|
 |
A.P.Wojtovich,
and
P.S.Brookes
(2009).
The complex II inhibitor atpenin A5 protects against cardiac ischemia-reperfusion injury via activation of mitochondrial KATP channels.
|
| |
Basic Res Cardiol,
104,
121-129.
|
 |
|
|
|
|
 |
J.Morales,
T.Mogi,
S.Mineki,
E.Takashima,
R.Mineki,
H.Hirawake,
K.Sakamoto,
S.Omura,
and
K.Kita
(2009).
Novel mitochondrial complex II isolated from Trypanosoma cruzi is composed of 12 peptides including a heterodimeric Ip subunit.
|
| |
J Biol Chem,
284,
7255-7263.
|
 |
|
|
|
|
 |
J.Ruprecht,
V.Yankovskaya,
E.Maklashina,
S.Iwata,
and
G.Cecchini
(2009).
Structure of Escherichia coli succinate:quinone oxidoreductase with an occupied and empty quinone-binding site.
|
| |
J Biol Chem,
284,
29836-29846.
|
 |
|
PDB codes:
|
 |
|
|
|
|
|
 |
M.Ohtawa,
S.Ogihara,
K.Sugiyama,
K.Shiomi,
Y.Harigaya,
T.Nagamitsu,
and
S.Omura
(2009).
Enantioselective total synthesis of atpenin A5.
|
| |
J Antibiot (Tokyo),
62,
289-294.
|
 |
|
|
|
|
 |
M.S.Albury,
C.Elliott,
and
A.L.Moore
(2009).
Towards a structural elucidation of the alternative oxidase in plants.
|
| |
Physiol Plant,
137,
316-327.
|
 |
|
|
|
|
 |
S.B.Choi,
Y.M.Normi,
and
H.A.Wahab
(2009).
Why hypothetical protein KPN00728 of Klebsiella pneumoniae should be classified as chain C of succinate dehydrogenase?
|
| |
Protein J,
28,
415-427.
|
 |
|
|
|
|
 |
H.F.Avenot,
A.Sellam,
G.Karaoglanidis,
and
T.J.Michailides
(2008).
Characterization of mutations in the iron-sulphur subunit of succinate dehydrogenase correlating with Boscalid resistance in Alternaria alternata from California pistachio.
|
| |
Phytopathology,
98,
736-742.
|
 |
|
|
|
|
 |
M.Jormakka,
K.Yokoyama,
T.Yano,
M.Tamakoshi,
S.Akimoto,
T.Shimamura,
P.Curmi,
and
S.Iwata
(2008).
Molecular mechanism of energy conservation in polysulfide respiration.
|
| |
Nat Struct Mol Biol,
15,
730-737.
|
 |
|
PDB codes:
|
 |
|
|
|
|
|
 |
R.J.Naftalin,
N.Green,
and
P.Cunningham
(2007).
Lactose permease H+-lactose symporter: mechanical switch or Brownian ratchet?
|
| |
Biophys J,
92,
3474-3491.
|
 |
|
|
|
|
 |
J.Zhang,
F.E.Frerman,
and
J.J.Kim
(2006).
Structure of electron transfer flavoprotein-ubiquinone oxidoreductase and electron transfer to the mitochondrial ubiquinone pool.
|
| |
Proc Natl Acad Sci U S A,
103,
16212-16217.
|
 |
|
PDB codes:
|
 |
|
|
 |
 |
|
The most recent references are shown first.
Citation data come partly from CiteXplore and partly
from an automated harvesting procedure. Note that this is likely to be
only a partial list as not all journals are covered by
either method. However, we are continually building up the citation data
so more and more references will be included with time.
Where a reference describes a PDB structure, the PDB
codes are
shown on the right.
|
 |
| |

