 |
PDBsum entry 1zh1

|
|
|

|
 |

|

|

|

|

|

|

|

|

|

|

|

|

|

|
 |

|

|

|
 |
 |
 |
 |
 |
 |
 |
 |
 |
 |
 |
 |
 |
 |
 |
 |
 |
 |

|

|

|

|

|

|

|

|

|
|
Metal binding protein
|
PDB id
|
|

|

|
1zh1
|

|

|

|

|

|

|

|

|

|

|

|

|

|

|

|

|

|

|

|
|
 |
Contents |
 |
|
|

|

|
|
|
|
|
|
|
|
* Residue conservation analysis
|
|
|
|
 |
|
|
 |
 |
 |
 |
Enzyme class 1:
|
 |
E.C.2.7.7.48
- RNA-directed Rna polymerase.
|
|
 |
 |
 |
 |
 |
Reaction:
|
 |
RNA(n) + a ribonucleoside 5'-triphosphate = RNA(n+1) + diphosphate
|
 |
 |
 |
 |
 |
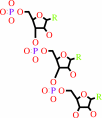 RNA(n)
RNA(n)
|
+
|
ribonucleoside 5'-triphosphate
|
=
|
RNA(n+1)
|
+
|
 diphosphate
diphosphate
|
|
 |
 |
 |
 |
 |
 |
 |
 |
Enzyme class 2:
|
 |
E.C.3.4.21.98
- hepacivirin.
|
|
 |
 |
 |
 |
 |
Reaction:
|
 |
Hydrolysis of four peptide bonds in the viral precursor polyprotein, commonly with Asp or Glu in the P6 position, Cys or Thr in P1 and Ser or Ala in P1'.
|
 |
 |
 |
 |
 |
Enzyme class 3:
|
 |
E.C.3.4.22.-
- ?????
|
|
 |
 |
 |
 |
 |
Enzyme class 4:
|
 |
E.C.3.6.1.15
- nucleoside-triphosphate phosphatase.
|
|
 |
 |
 |
 |
 |
Reaction:
|
 |
a ribonucleoside 5'-triphosphate + H2O = a ribonucleoside 5'-diphosphate + phosphate + H+
|
 |
 |
 |
 |
 |
ribonucleoside 5'-triphosphate
|
+
|
H2O
|
=
|
ribonucleoside 5'-diphosphate
|
+
|
 phosphate
phosphate
|
+
|
H(+)
|
|
 |
 |
 |
 |
 |
 |
 |
 |
Enzyme class 5:
|
 |
E.C.3.6.4.13
- Rna helicase.
|
|
 |
 |
 |
 |
 |
Reaction:
|
 |
ATP + H2O = ADP + phosphate + H+
|
 |
 |
 |
 |
 |
 ATP
ATP
|
+
|
H2O
|
=
|
 ADP
ADP
|
+
|
 phosphate
phosphate
|
+
|
H(+)
|
|
 |
 |
 |
 |
 |
 |
 |
 |
 |
 |
|
Note, where more than one E.C. class is given (as above), each may
correspond to a different protein domain or, in the case of polyprotein
precursors, to a different mature protein.
|
|
 |
|
Molecule diagrams generated from .mol files obtained from the
KEGG ftp site
|
|
 |
 |
 |
 |
 |
 |
 |
 |
 |
 |
 |
 |
 |
 |
 |

|

|

|
| |
|
|
| |
|
DOI no:
|
Nature
435:374-379
(2005)
|
|
PubMed id:
|
|
|
|

|
| |
|
Structure of the zinc-binding domain of an essential component of the hepatitis C virus replicase.
|
|
T.L.Tellinghuisen,
J.Marcotrigiano,
C.M.Rice.
|
|
|

|
| |
ABSTRACT
|
|

|
| |

|
Hepatitis C virus (HCV) is a human pathogen affecting nearly 3% of the world's
population. Chronic infections can lead to cirrhosis and liver cancer. The RNA
replication machine of HCV is a multi-subunit membrane-associated complex. The
non-structural protein NS5A is an active component of HCV replicase, as well as
a pivotal regulator of replication and a modulator of cellular processes ranging
from innate immunity to dysregulated cell growth. NS5A is a large phosphoprotein
(56-58 kDa) with an amphipathic alpha-helix at its amino terminus that promotes
membrane association. After this helix region, NS5A is organized into three
domains. The N-terminal domain (domain I) coordinates a single zinc atom per
protein molecule. Mutations disrupting either the membrane anchor or zinc
binding of NS5A are lethal for RNA replication. However, probing the role of
NS5A in replication has been hampered by a lack of structural information about
this multifunctional protein. Here we report the structure of NS5A domain I at
2.5-A resolution, which contains a novel fold, a new zinc-coordination motif and
a disulphide bond. We use molecular surface analysis to suggest the location of
protein-, RNA- and membrane-interaction sites.

|

|
|
|

|
| |
Selected figure(s)
|
|

|
| |
 |
 |

|
 |

|
 |
Figure 1.
Figure 1: An overview of the NS5A domain I structure. a,
Schematic of HCV genome organization and the domain structure of
the NS5A protein. The portion of domain I in presented in this
crystal structure is indicated by the red bar. C, capsid
protein; E1 and E2, envelope glycoproteins 1 and 2
(respectively); 4A, NS4A protein. b, Ribbon diagram of the
structure of domain I. The polypeptide chain is coloured from
the N terminus (blue) to C terminus (red). The coordinated zinc
atom is shown in yellow. The C-terminal disulphide bond is shown
in blue. c, A 180° rotation showing the 'back' of domain I. d, A
90° rotation showing the 'top-down' view of domain I. e, Domain
I topology organization model.
|
 |
Figure 4.
Figure 4: The NS5A domain I dimer reveals potential interaction
surfaces. a, Ribbon diagrams of three rotations of the domain
I dimer. b, Surface-potential plots of the domain I dimer, with
views corresponding to those shown in a. Analysis of images in a
and b shows that the dimer creates a relatively flat, basic
surface near the N terminus and a large groove between the two
subdomain IB regions. c, Model of NS5A position relative to the
endoplasmic reticulum membrane. The location of the conserved
surface in Fig. 3b is indicated. Helices are from the recent
structure of the NS5A N-terminal helix8.
|
 |
|
|

|
| |
The above figures are
reprinted
from an Open Access publication published by Macmillan Publishers Ltd:
Nature
(2005,
435,
374-379)
copyright 2005.
|
|
| |
Figures were
selected
by an automated process.
|
|

|

|
|
 |
 |
|
 |
 |
 |
 |
 |
 |
 |
 |
 |
|
Literature references that cite this PDB file's key reference
|
|
 |
| |
PubMed id
|
 |
Reference
|
 |
|
|
|
 |
C.Lee,
H.Ma,
J.Q.Hang,
V.Leveque,
E.H.Sklan,
M.Elazar,
K.Klumpp,
and
J.S.Glenn
(2011).
The hepatitis C virus NS5A inhibitor (BMS-790052) alters the subcellular localization of the NS5A non-structural viral protein.
|
| |
Virology,
414,
10-18.
|
 |
|
|
|
|
 |
T.Suzuki
(2011).
Assembly of hepatitis C virus particles.
|
| |
Microbiol Immunol,
55,
12-18.
|
 |
|
|
|
|
 |
A.Nordle Gilliver,
S.Griffin,
and
M.Harris
(2010).
Identification of a novel phosphorylation site in hepatitis C virus NS5A.
|
| |
J Gen Virol,
91,
2428-2432.
|
 |
|
|
|
|
 |
C.L.Murray,
and
C.M.Rice
(2010).
Hepatitis C: An unsuspected drug target.
|
| |
Nature,
465,
42-44.
|
 |
|
|
|
|
 |
C.M.Lange,
C.Sarrazin,
and
S.Zeuzem
(2010).
Review article: specifically targeted anti-viral therapy for hepatitis C - a new era in therapy.
|
| |
Aliment Pharmacol Ther,
32,
14-28.
|
 |
|
|
|
|
 |
C.M.Lange,
M.von Wagner,
J.Bojunga,
T.Berg,
H.Farnik,
A.Hassler,
C.Sarrazin,
E.Herrmann,
and
S.Zeuzem
(2010).
Serum lipids in European chronic HCV genotype 1 patients during and after treatment with pegylated interferon-α-2a and ribavirin.
|
| |
Eur J Gastroenterol Hepatol,
22,
1303-1307.
|
 |
|
|
|
|
 |
F.Fernandes,
I.U.Ansari,
and
R.Striker
(2010).
cyclosporine inhibits a direct interaction between cyclophilins and hepatitis C NS5A.
|
| |
PLoS One,
5,
e9815.
|
 |
|
|
|
|
 |
J.A.Lemm,
D.O'Boyle,
M.Liu,
P.T.Nower,
R.Colonno,
M.S.Deshpande,
L.B.Snyder,
S.W.Martin,
D.R.St Laurent,
M.H.Serrano-Wu,
J.L.Romine,
N.A.Meanwell,
and
M.Gao
(2010).
Identification of hepatitis C virus NS5A inhibitors.
|
| |
J Virol,
84,
482-491.
|
 |
|
|
|
|
 |
L.Coelmont,
X.Hanoulle,
U.Chatterji,
C.Berger,
J.Snoeck,
M.Bobardt,
P.Lim,
I.Vliegen,
J.Paeshuyse,
G.Vuagniaux,
A.M.Vandamme,
R.Bartenschlager,
P.Gallay,
G.Lippens,
and
J.Neyts
(2010).
DEB025 (Alisporivir) inhibits hepatitis C virus replication by preventing a cyclophilin A induced cis-trans isomerisation in domain II of NS5A.
|
| |
PLoS One,
5,
e13687.
|
 |
|
|
|
|
 |
M.A.Zahoor,
D.Yamane,
Y.M.Mohamed,
Y.Suda,
K.Kobayashi,
K.Kato,
Y.Tohya,
and
H.Akashi
(2010).
Bovine viral diarrhea virus non-structural protein 5A interacts with NIK- and IKKbeta-binding protein.
|
| |
J Gen Virol,
91,
1939-1948.
|
 |
|
|
|
|
 |
M.Gao,
R.E.Nettles,
M.Belema,
L.B.Snyder,
V.N.Nguyen,
R.A.Fridell,
M.H.Serrano-Wu,
D.R.Langley,
J.H.Sun,
D.R.O'Boyle,
J.A.Lemm,
C.Wang,
J.O.Knipe,
C.Chien,
R.J.Colonno,
D.M.Grasela,
N.A.Meanwell,
and
L.G.Hamann
(2010).
Chemical genetics strategy identifies an HCV NS5A inhibitor with a potent clinical effect.
|
| |
Nature,
465,
96.
|
 |
|
|
|
|
 |
R.A.Fridell,
D.Qiu,
C.Wang,
L.Valera,
and
M.Gao
(2010).
Resistance analysis of the hepatitis C virus NS5A inhibitor BMS-790052 in an in vitro replicon system.
|
| |
Antimicrob Agents Chemother,
54,
3641-3650.
|
 |
|
|
|
|
 |
S.Margeridon-Thermet,
and
R.W.Shafer
(2010).
Comparison of the Mechanisms of Drug Resistance among HIV, Hepatitis B, and Hepatitis C.
|
| |
Viruses,
2,
2696-2739.
|
 |
|
|
|
|
 |
T.L.Foster,
T.Belyaeva,
N.J.Stonehouse,
A.R.Pearson,
and
M.Harris
(2010).
All three domains of the hepatitis C virus nonstructural NS5A protein contribute to RNA binding.
|
| |
J Virol,
84,
9267-9277.
|
 |
|
|
|
|
 |
W.Hou,
Q.Tian,
J.Zheng,
and
H.L.Bonkovsky
(2010).
Zinc mesoporphyrin induces rapid proteasomal degradation of hepatitis C nonstructural 5A protein in human hepatoma cells.
|
| |
Gastroenterology,
138,
1909-1919.
|
 |
|
|
|
|
 |
Y.C.Chen,
W.C.Su,
J.Y.Huang,
T.C.Chao,
K.S.Jeng,
K.Machida,
and
M.M.Lai
(2010).
Polo-like kinase 1 is involved in hepatitis C virus replication by hyperphosphorylating NS5A.
|
| |
J Virol,
84,
7983-7993.
|
 |
|
|
|
|
 |
H.Kukihara,
K.Moriishi,
S.Taguwa,
H.Tani,
T.Abe,
Y.Mori,
T.Suzuki,
T.Fukuhara,
A.Taketomi,
Y.Maehara,
and
Y.Matsuura
(2009).
Human VAP-C negatively regulates hepatitis C virus propagation.
|
| |
J Virol,
83,
7959-7969.
|
 |
|
|
|
|
 |
H.Tang,
and
H.Grisé
(2009).
Cellular and molecular biology of HCV infection and hepatitis.
|
| |
Clin Sci (Lond),
117,
49-65.
|
 |
|
|
|
|
 |
M.Hughes,
S.Griffin,
and
M.Harris
(2009).
Domain III of NS5A contributes to both RNA replication and assembly of hepatitis C virus particles.
|
| |
J Gen Virol,
90,
1329-1334.
|
 |
|
|
|
|
 |
M.M.El Hefnawi,
W.H.El Behaidy,
A.A.Youssif,
A.Z.Ghalwash,
L.A.El Housseiny,
and
S.Zada
(2009).
Natural genetic engineering of hepatitis C virus NS5A for immune system counterattack.
|
| |
Ann N Y Acad Sci,
1178,
173-185.
|
 |
|
|
|
|
 |
N.Kato,
K.Abe,
K.Mori,
Y.Ariumi,
H.Dansako,
and
M.Ikeda
(2009).
Genetic variability and diversity of intracellular genome-length hepatitis C virus RNA in long-term cell culture.
|
| |
Arch Virol,
154,
77-85.
|
 |
|
|
|
|
 |
R.A.Love,
O.Brodsky,
M.J.Hickey,
P.A.Wells,
and
C.N.Cronin
(2009).
Crystal structure of a novel dimeric form of NS5A domain I protein from hepatitis C virus.
|
| |
J Virol,
83,
4395-4403.
|
 |
|
PDB codes:
|
 |
|
|
|
|
|
 |
S.Taguwa,
H.Kambara,
H.Omori,
H.Tani,
T.Abe,
Y.Mori,
T.Suzuki,
T.Yoshimori,
K.Moriishi,
and
Y.Matsuura
(2009).
Cochaperone activity of human butyrate-induced transcript 1 facilitates hepatitis C virus replication through an Hsp90-dependent pathway.
|
| |
J Virol,
83,
10427-10436.
|
 |
|
|
|
|
 |
V.Meier,
and
G.Ramadori
(2009).
Hepatitis C virus virology and new treatment targets.
|
| |
Expert Rev Anti Infect Ther,
7,
329-350.
|
 |
|
|
|
|
 |
X.Hanoulle,
A.Badillo,
J.M.Wieruszeski,
D.Verdegem,
I.Landrieu,
R.Bartenschlager,
F.Penin,
and
G.Lippens
(2009).
Hepatitis C virus NS5A protein is a substrate for the peptidyl-prolyl cis/trans isomerase activity of cyclophilins A and B.
|
| |
J Biol Chem,
284,
13589-13601.
|
 |
|
|
|
|
 |
Y.Bungyoku,
I.Shoji,
T.Makine,
T.Adachi,
K.Hayashida,
M.Nagano-Fujii,
Y.H.Ide,
L.Deng,
and
H.Hotta
(2009).
Efficient production of infectious hepatitis C virus with adaptive mutations in cultured hepatoma cells.
|
| |
J Gen Virol,
90,
1681-1691.
|
 |
|
|
|
|
 |
Y.Zhou,
W.P.Tzeng,
Y.Ye,
Y.Huang,
S.Li,
Y.Chen,
T.K.Frey,
and
J.J.Yang
(2009).
A cysteine-rich metal-binding domain from rubella virus non-structural protein is essential for viral protease activity and virus replication.
|
| |
Biochem J,
417,
477-483.
|
 |
|
|
|
|
 |
G.Cheng,
A.Montero,
P.Gastaminza,
C.Whitten-Bauer,
S.F.Wieland,
M.Isogawa,
B.Fredericksen,
S.Selvarajah,
P.A.Gallay,
M.R.Ghadiri,
and
F.V.Chisari
(2008).
A virocidal amphipathic {alpha}-helical peptide that inhibits hepatitis C virus infection in vitro.
|
| |
Proc Natl Acad Sci U S A,
105,
3088-3093.
|
 |
|
|
|
|
 |
H.Shelton,
and
M.Harris
(2008).
Hepatitis C virus NS5A protein binds the SH3 domain of the Fyn tyrosine kinase with high affinity: mutagenic analysis of residues within the SH3 domain that contribute to the interaction.
|
| |
Virol J,
5,
24.
|
 |
|
|
|
|
 |
N.A.Cannon,
M.J.Donlin,
X.Fan,
R.Aurora,
and
J.E.Tavis
(2008).
Hepatitis C virus diversity and evolution in the full open-reading frame during antiviral therapy.
|
| |
PLoS ONE,
3,
e2123.
|
 |
|
|
|
|
 |
N.Appel,
M.Zayas,
S.Miller,
J.Krijnse-Locker,
T.Schaller,
P.Friebe,
S.Kallis,
U.Engel,
and
R.Bartenschlager
(2008).
Essential role of domain III of nonstructural protein 5A for hepatitis C virus infectious particle assembly.
|
| |
PLoS Pathog,
4,
e1000035.
|
 |
|
|
|
|
 |
P.Targett-Adams,
S.Boulant,
and
J.McLauchlan
(2008).
Visualization of double-stranded RNA in cells supporting hepatitis C virus RNA replication.
|
| |
J Virol,
82,
2182-2195.
|
 |
|
|
|
|
 |
S.Inubushi,
M.Nagano-Fujii,
K.Kitayama,
M.Tanaka,
C.An,
H.Yokozaki,
H.Yamamura,
H.Nuriya,
M.Kohara,
K.Sada,
and
H.Hotta
(2008).
Hepatitis C virus NS5A protein interacts with and negatively regulates the non-receptor protein tyrosine kinase Syk.
|
| |
J Gen Virol,
89,
1231-1242.
|
 |
|
|
|
|
 |
T.L.Tellinghuisen,
K.L.Foss,
J.C.Treadaway,
and
C.M.Rice
(2008).
Identification of residues required for RNA replication in domains II and III of the hepatitis C virus NS5A protein.
|
| |
J Virol,
82,
1073-1083.
|
 |
|
|
|
|
 |
T.L.Tellinghuisen,
K.L.Foss,
and
J.Treadaway
(2008).
Regulation of hepatitis C virion production via phosphorylation of the NS5A protein.
|
| |
PLoS Pathog,
4,
e1000032.
|
 |
|
|
|
|
 |
T.Masaki,
R.Suzuki,
K.Murakami,
H.Aizaki,
K.Ishii,
A.Murayama,
T.Date,
Y.Matsuura,
T.Miyamura,
T.Wakita,
and
T.Suzuki
(2008).
Interaction of hepatitis C virus nonstructural protein 5A with core protein is critical for the production of infectious virus particles.
|
| |
J Virol,
82,
7964-7976.
|
 |
|
|
|
|
 |
T.Okamoto,
H.Omori,
Y.Kaname,
T.Abe,
Y.Nishimura,
T.Suzuki,
T.Miyamura,
T.Yoshimori,
K.Moriishi,
and
Y.Matsuura
(2008).
A single-amino-acid mutation in hepatitis C virus NS5A disrupting FKBP8 interaction impairs viral replication.
|
| |
J Virol,
82,
3480-3489.
|
 |
|
|
|
|
 |
T.Suzuki,
T.Masaki,
and
H.Aizaki
(2008).
[Involvement of nonstructural protein 5A and lipids on production of hepatitis C virus particles]
|
| |
Uirusu,
58,
199-205.
|
 |
|
|
|
|
 |
V.Arumugaswami,
R.Remenyi,
V.Kanagavel,
E.Y.Sue,
T.Ngoc Ho,
C.Liu,
V.Fontanes,
A.Dasgupta,
and
R.Sun
(2008).
High-resolution functional profiling of hepatitis C virus genome.
|
| |
PLoS Pathog,
4,
e1000182.
|
 |
|
|
|
|
 |
Y.Hirata,
M.Sudoh,
and
M.Kohara
(2008).
[Suppression of hepatitis C virus with the reagent targetting host factors]
|
| |
Uirusu,
58,
207-213.
|
 |
|
|
|
|
 |
A.Wohnsland,
W.P.Hofmann,
and
C.Sarrazin
(2007).
Viral determinants of resistance to treatment in patients with hepatitis C.
|
| |
Clin Microbiol Rev,
20,
23-38.
|
 |
|
|
|
|
 |
B.D.Lindenbach,
B.M.Prágai,
R.Montserret,
R.K.Beran,
A.M.Pyle,
F.Penin,
and
C.M.Rice
(2007).
The C terminus of hepatitis C virus NS4A encodes an electrostatic switch that regulates NS5A hyperphosphorylation and viral replication.
|
| |
J Virol,
81,
8905-8918.
|
 |
|
|
|
|
 |
K.L.Maxwell,
and
L.Frappier
(2007).
Viral proteomics.
|
| |
Microbiol Mol Biol Rev,
71,
398-411.
|
 |
|
|
|
|
 |
M.Binder,
D.Quinkert,
O.Bochkarova,
R.Klein,
N.Kezmic,
R.Bartenschlager,
and
V.Lohmann
(2007).
Identification of determinants involved in initiation of hepatitis C virus RNA synthesis by using intergenotypic replicase chimeras.
|
| |
J Virol,
81,
5270-5283.
|
 |
|
|
|
|
 |
T.L.Tellinghuisen,
M.J.Evans,
T.von Hahn,
S.You,
and
C.M.Rice
(2007).
Studying hepatitis C virus: making the best of a bad virus.
|
| |
J Virol,
81,
8853-8867.
|
 |
|
|
|
|
 |
T.Suzuki,
K.Ishii,
H.Aizaki,
and
T.Wakita
(2007).
Hepatitis C viral life cycle.
|
| |
Adv Drug Deliv Rev,
59,
1200-1212.
|
 |
|
|
|
|
 |
G.Szabo
(2006).
Hepatitis C virus NS5A protein--a master regulator?
|
| |
Gastroenterology,
130,
995-999.
|
 |
|
|
|
|
 |
J.R.Mesters,
J.Tan,
and
R.Hilgenfeld
(2006).
Viral enzymes.
|
| |
Curr Opin Struct Biol,
16,
776-786.
|
 |
|
|
|
|
 |
K.Yuasa,
A.Naganuma,
K.Sato,
M.Ikeda,
N.Kato,
H.Takagi,
and
M.Mori
(2006).
Zinc is a negative regulator of hepatitis C virus RNA replication.
|
| |
Liver Int,
26,
1111-1118.
|
 |
|
|
|
|
 |
M.Quintavalle,
S.Sambucini,
C.Di Pietro,
R.De Francesco,
and
P.Neddermann
(2006).
The alpha isoform of protein kinase CKI is responsible for hepatitis C virus NS5A hyperphosphorylation.
|
| |
J Virol,
80,
11305-11312.
|
 |
|
|
|
|
 |
N.Appel,
T.Schaller,
F.Penin,
and
R.Bartenschlager
(2006).
From structure to function: new insights into hepatitis C virus RNA replication.
|
| |
J Biol Chem,
281,
9833-9836.
|
 |
|
|
|
|
 |
T.L.Tellinghuisen,
M.S.Paulson,
and
C.M.Rice
(2006).
The NS5A protein of bovine viral diarrhea virus contains an essential zinc-binding site similar to that of the hepatitis C virus NS5A protein.
|
| |
J Virol,
80,
7450-7458.
|
 |
|
|
|
|
 |
T.Okamoto,
Y.Nishimura,
T.Ichimura,
K.Suzuki,
T.Miyamura,
T.Suzuki,
K.Moriishi,
and
Y.Matsuura
(2006).
Hepatitis C virus RNA replication is regulated by FKBP8 and Hsp90.
|
| |
EMBO J,
25,
5015-5025.
|
 |
|
|
|
|
 |
V.Brass,
D.Moradpour,
and
H.E.Blum
(2006).
Molecular virology of hepatitis C virus (HCV): 2006 update.
|
| |
Int J Med Sci,
3,
29-34.
|
 |
|
|
|
|
 |
B.D.Lindenbach,
and
C.M.Rice
(2005).
Unravelling hepatitis C virus replication from genome to function.
|
| |
Nature,
436,
933-938.
|
 |
|
|
|
|
 |
C.Seeger
(2005).
Salient molecular features of hepatitis C virus revealed.
|
| |
Trends Microbiol,
13,
528-534.
|
 |
|
|
|
|
 |
I.Hamamoto,
Y.Nishimura,
T.Okamoto,
H.Aizaki,
M.Liu,
Y.Mori,
T.Abe,
T.Suzuki,
M.M.Lai,
T.Miyamura,
K.Moriishi,
and
Y.Matsuura
(2005).
Human VAP-B is involved in hepatitis C virus replication through interaction with NS5A and NS5B.
|
| |
J Virol,
79,
13473-13482.
|
 |
|
|
|
|
 |
L.Huang,
J.Hwang,
S.D.Sharma,
M.R.Hargittai,
Y.Chen,
J.J.Arnold,
K.D.Raney,
and
C.E.Cameron
(2005).
Hepatitis C virus nonstructural protein 5A (NS5A) is an RNA-binding protein.
|
| |
J Biol Chem,
280,
36417-36428.
|
 |
|
 |
 |
|
The most recent references are shown first.
Citation data come partly from CiteXplore and partly
from an automated harvesting procedure. Note that this is likely to be
only a partial list as not all journals are covered by
either method. However, we are continually building up the citation data
so more and more references will be included with time.
Where a reference describes a PDB structure, the PDB
codes are
shown on the right.
|
 |

