 |
PDBsum entry 1mj4

|
|
|

|
 |

|

|

|

|

|

|

|

|

|

|

|

|

|
 |

|

|

|
 |
 |
 |
 |
 |
 |
 |
 |
 |
 |
 |
 |
 |
 |
 |
 |

|

|

|

|

|

|

|

|

|
|
Oxidoreductase
|
PDB id
|
|

|

|
1mj4
|

|

|

|

|

|

|

|

|

|

|

|

|

|

|

|

|

|

|

|
|
 |
Contents |
 |
|
|

|

|
|
|
|
|
|
|
|
* Residue conservation analysis
|
|
|
|
|
References listed in PDB file
|
 |
|
Key reference
|
 |
|
Title
|
 |
The 1.2 a structure of the human sulfite oxidase cytochrome b(5) domain.
|
 |
|
Authors
|
 |
M.J.Rudolph,
J.L.Johnson,
K.V.Rajagopalan,
C.Kisker.
|
 |
|
Ref.
|
 |
Acta Crystallogr D Biol Crystallogr, 2003,
59,
1183-1191.
[DOI no: ]
|
 |
|
PubMed id
|
 |
|
 |
 |
|
Abstract
|
 |
|
The molybdenum- and iron-containing enzyme sulfite oxidase catalyzes the
physiologically vital oxidation of sulfite to sulfate. Sulfite oxidase contains
three domains: an N-terminal cytochrome b(5) domain, a central domain harboring
the molybdenum cofactor (Moco) and a C-terminal dimerization domain. Oxidation
of the substrate sulfite is coupled to the transfer of two electrons to the
molybdenum cofactor. Subsequently, these electrons are passed on, one at a time,
to the b(5) heme of sulfite oxidase and from there to the soluble electron
carrier cytochrome c. The crystal structure of the oxidized human sulfite
oxidase cytochrome b(5) domain has been determined at 1.2 A resolution and has
been refined to a crystallographic R factor of 0.107 (R(free) = 0.137). A
comparison of this structure with other b(5)-type cytochromes reveals distinct
structural features present in the sulfite oxidase b(5) domain which promote
optimal electron transport between the Moco of sulfite oxidase and the heme of
cytochrome c.
|
 |
 |
 |

|
 |

|
 |
Figure 1.
Figure 1 Sulfite oxidase-catalyzed reaction. The reaction
consists of the oxidation of sulfite to sulfate coupled with the
subsequent reduction of two equivalents of ferricytochrome c to
ferrocytochrome c.
|
 |
Figure 8.
Figure 8 Representation of the HSO b[5] (a) and CYT b[5] (b)
heme axial ligand environments. The heme is shown in all-bonds
representation, with the iron in green. The axial histidines are
in all-bonds representation, with the red dots depicting the
interactions between the Fe atom and the N  2
atoms of the axial histidines as well as the hydrogen bonds
between the carbonyl O atoms and the N 2
atoms of the axial histidines as well as the hydrogen bonds
between the carbonyl O atoms and the N 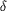 1
atoms from the axial histidines. 1
atoms from the axial histidines.
|
 |
|
 |
 |
|
The above figures are
reprinted
by permission from the IUCr:
Acta Crystallogr D Biol Crystallogr
(2003,
59,
1183-1191)
copyright 2003.
|
 |
|

|
|
|
 |

