 |
PDBsum entry 1ht5

|
|
|

|
 |

|

|

|

|

|

|

|

|

|

|

|

|

|

|

|

|

|

|
 |

|

|

|
 |
 |
 |
 |
 |
 |
 |
 |
 |
 |
 |
 |
 |
 |
 |
 |
 |
 |
 |
 |

|

|

|

|

|

|

|

|

|
|
Oxidoreductase
|
PDB id
|
|

|

|
1ht5
|

|

|

|

|

|

|

|

|

|

|

|

|

|

|

|

|

|

|

|
|
 |
Contents |
 |
|
|

|

|
|
|
|
|
|
|
|
* Residue conservation analysis
|
|
|
|
 |
|
|
 |
 |
 |
 |
Enzyme class:
|
 |
E.C.1.14.99.1
- prostaglandin-endoperoxide synthase.
|
|
 |
 |
 |
 |
 |
Reaction:
|
 |
(5Z,8Z,11Z,14Z)-eicosatetraenoate + AH2 + 2 O2 = prostaglandin H2 + A + H2O
|
 |
 |
 |
 |
 |
(5Z,8Z,11Z,14Z)-eicosatetraenoate
|
+
|
AH2
|
+
|
 2
×
O2
2
×
O2
|
=
|
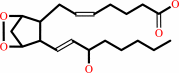 prostaglandin H2
prostaglandin H2
|
+
|
|
+
|
H2O
Bound ligand (Het Group name = )
matches with 51.11% similarity
|
|
 |
 |
 |
 |
 |
 |
 |
 |
 |
 |
|
Molecule diagrams generated from .mol files obtained from the
KEGG ftp site
|
|
 |
 |
 |
 |
 |
 |
 |
 |
 |
 |
 |
 |
 |
 |
 |

|

|

|
| |
|
|
| |
|
|
Biochemistry
40:5172-5180
(2001)
|
|
PubMed id:
|
|
|
|

|
| |
|
Structural analysis of NSAID binding by prostaglandin H2 synthase: time-dependent and time-independent inhibitors elicit identical enzyme conformations.
|
|
B.S.Selinsky,
K.Gupta,
C.T.Sharkey,
P.J.Loll.
|
|
|

|
| |
ABSTRACT
|
|

|
| |

|
Nonsteroidal antiinflammatory drugs (NSAIDs) block prostanoid biosynthesis by
inhibiting prostaglandin H(2) synthase (EC 1.14.99.1). NSAIDs are either rapidly
reversible competitive inhibitors or slow tight-binding inhibitors of this
enzyme. These different modes of inhibition correlate with clinically important
differences in isoform selectivity. Hypotheses have been advanced to explain the
different inhibition kinetics, but no structural data have been available to
test them. We present here crystal structures of prostaglandin H(2) synthase-1
in complex with the inhibitors ibuprofen, methyl flurbiprofen, flurbiprofen, and
alclofenac at resolutions ranging from 2.6 to 2.75 A. These structures allow
direct comparison of enzyme complexes with reversible competitive inhibitors
(ibuprofen and methyl flurbiprofen) and slow tight-binding inhibitors
(alclofenac and flurbiprofen). The four inhibitors bind to the same site and
adopt similar conformations. In all four complexes, the enzyme structure is
essentially unchanged, exhibiting only minimal differences in the inhibitor
binding site. These results argue strongly against hypotheses that explain the
difference between slow tight-binding and fast reversible competitive inhibition
by invoking global conformational differences or different inhibitor binding
sites. Instead, they suggest that the different apparent modes of NSAID binding
may result from differences in the speed and efficiency with which inhibitors
can perturb the hydrogen bonding network around Arg-120 and Tyr-355.

|

|
|
|

|

|
|
 |
 |
|
 |
 |
 |
 |
 |
 |
 |
 |
 |
|
Literature references that cite this PDB file's key reference
|
|
 |
| |
PubMed id
|
 |
Reference
|
 |
|
|
|
 |
J.M.Lü,
C.E.Rogge,
G.Wu,
R.J.Kulmacz,
W.A.van der Donk,
and
A.L.Tsai
(2011).
Cyclooxygenase reaction mechanism of PGHS--evidence for a reversible transition between a pentadienyl radical and a new tyrosyl radical by nitric oxide trapping.
|
| |
J Inorg Biochem,
105,
356-365.
|
 |
|
|
|
|
 |
A.Régnier,
E.Vicaut,
and
S.Mraovitch
(2010).
Aggravation of seizure-associated microvascular injuries by ibuprofen may involve multiple pathways.
|
| |
Epilepsia,
51,
2412-2422.
|
 |
|
|
|
|
 |
A.Tripathi,
and
G.E.Kellogg
(2010).
A novel and efficient tool for locating and characterizing protein cavities and binding sites.
|
| |
Proteins,
78,
825-842.
|
 |
|
|
|
|
 |
C.E.Cassidy,
and
W.N.Setzer
(2010).
Cancer-relevant biochemical targets of cytotoxic Lonchocarpus flavonoids: a molecular docking analysis.
|
| |
J Mol Model,
16,
311-326.
|
 |
|
|
|
|
 |
S.Bouaziz-Terrachet,
A.Toumi-Maouche,
B.Maouche,
and
S.Taïri-Kellou
(2010).
Modeling the binding modes of stilbene analogs to cyclooxygenase-2: a molecular docking study.
|
| |
J Mol Model,
16,
1919-1929.
|
 |
|
|
|
|
 |
V.Limongelli,
M.Bonomi,
L.Marinelli,
F.L.Gervasio,
A.Cavalli,
E.Novellino,
and
M.Parrinello
(2010).
Molecular basis of cyclooxygenase enzymes (COXs) selective inhibition.
|
| |
Proc Natl Acad Sci U S A,
107,
5411-5416.
|
 |
|
|
|
|
 |
C.E.Rogge,
W.Liu,
R.J.Kulmacz,
and
A.L.Tsai
(2009).
Peroxide-induced radical formation at TYR385 and TYR504 in human PGHS-1.
|
| |
J Inorg Biochem,
103,
912-922.
|
 |
|
|
|
|
 |
M.Bala,
C.N.Chin,
A.T.Logan,
T.Amin,
L.J.Marnett,
O.Boutaud,
and
J.A.Oates
(2008).
Acetylation of prostaglandin H2 synthases by aspirin is inhibited by redox cycling of the peroxidase.
|
| |
Biochem Pharmacol,
75,
1472-1481.
|
 |
|
|
|
|
 |
M.L.Vueba,
M.E.Pina,
and
L.A.Batista de Carvalho
(2008).
Conformational stability of ibuprofen: Assessed by DFT calculations and optical vibrational spectroscopy.
|
| |
J Pharm Sci,
97,
834-848.
|
 |
|
|
|
|
 |
Y.Tanrikulu,
and
G.Schneider
(2008).
Pseudoreceptor models in drug design: bridging ligand- and receptor-based virtual screening.
|
| |
Nat Rev Drug Discov,
7,
667-677.
|
 |
|
|
|
|
 |
A.Peretz,
N.Degani-Katzav,
M.Talmon,
E.Danieli,
A.Gopin,
E.Malka,
R.Nachman,
A.Raz,
D.Shabat,
and
B.Attali
(2007).
A tale of switched functions: from cyclooxygenase inhibition to m-channel modulation in new diphenylamine derivatives.
|
| |
PLoS ONE,
2,
e1332.
|
 |
|
|
|
|
 |
G.A.Gale,
K.Kirtikara,
P.Pittayakhajonwut,
S.Sivichai,
Y.Thebtaranonth,
C.Thongpanchang,
and
V.Vichai
(2007).
In search of cyclooxygenase inhibitors, anti-Mycobacterium tuberculosis and anti-malarial drugs from Thai flora and microbes.
|
| |
Pharmacol Ther,
115,
307-351.
|
 |
|
|
|
|
 |
C.E.Rogge,
B.Ho,
W.Liu,
R.J.Kulmacz,
and
A.L.Tsai
(2006).
Role of Tyr348 in Tyr385 radical dynamics and cyclooxygenase inhibitor interactions in prostaglandin H synthase-2.
|
| |
Biochemistry,
45,
523-532.
|
 |
|
|
|
|
 |
K.Gupta,
B.S.Selinsky,
and
P.J.Loll
(2006).
2.0 angstroms structure of prostaglandin H2 synthase-1 reconstituted with a manganese porphyrin cofactor.
|
| |
Acta Crystallogr D Biol Crystallogr,
62,
151-156.
|
 |
|
PDB code:
|
 |
|
|
|
|
|
 |
P.W.Fowler,
and
P.V.Coveney
(2006).
A computational protocol for the integration of the monotopic protein prostaglandin H2 synthase into a phospholipid bilayer.
|
| |
Biophys J,
91,
401-410.
|
 |
|
|
|
|
 |
S.Bingham,
P.J.Beswick,
D.E.Blum,
N.M.Gray,
and
I.P.Chessell
(2006).
The role of the cylooxygenase pathway in nociception and pain.
|
| |
Semin Cell Dev Biol,
17,
544-554.
|
 |
|
|
|
|
 |
Y.Yu,
J.Fan,
X.S.Chen,
D.Wang,
A.J.Klein-Szanto,
R.L.Campbell,
G.A.FitzGerald,
and
C.D.Funk
(2006).
Genetic model of selective COX2 inhibition reveals novel heterodimer signaling.
|
| |
Nat Med,
12,
699-704.
|
 |
|
|
|
|
 |
H.Park,
and
S.Lee
(2005).
Free energy perturbation approach to the critical assessment of selective cyclooxygenase-2 inhibitors.
|
| |
J Comput Aided Mol Des,
19,
17-31.
|
 |
|
|
|
|
 |
J.Aishima,
D.S.Russel,
L.J.Guibas,
P.D.Adams,
and
A.T.Brunger
(2005).
Automated crystallographic ligand building using the medial axis transform of an electron-density isosurface.
|
| |
Acta Crystallogr D Biol Crystallogr,
61,
1354-1363.
|
 |
|
|
|
|
 |
R.G.Huff,
E.Bayram,
H.Tan,
S.T.Knutson,
M.H.Knaggs,
A.B.Richon,
P.Santago,
and
J.S.Fetrow
(2005).
Chemical and structural diversity in cyclooxygenase protein active sites.
|
| |
Chem Biodivers,
2,
1533-1552.
|
 |
|
|
|
|
 |
D.A.Svistunenko,
and
C.E.Cooper
(2004).
A new method of identifying the site of tyrosyl radicals in proteins.
|
| |
Biophys J,
87,
582-595.
|
 |
|
|
|
|
 |
L.M.Szewczuk,
L.Forti,
L.A.Stivala,
and
T.M.Penning
(2004).
Resveratrol is a peroxidase-mediated inactivator of COX-1 but not COX-2: a mechanistic approach to the design of COX-1 selective agents.
|
| |
J Biol Chem,
279,
22727-22737.
|
 |
|
|
|
|
 |
R.Bertini,
M.Allegretti,
C.Bizzarri,
A.Moriconi,
M.Locati,
G.Zampella,
M.N.Cervellera,
V.Di Cioccio,
M.C.Cesta,
E.Galliera,
F.O.Martinez,
R.Di Bitondo,
G.Troiani,
V.Sabbatini,
G.D'Anniballe,
R.Anacardio,
J.C.Cutrin,
B.Cavalieri,
F.Mainiero,
R.Strippoli,
P.Villa,
M.Di Girolamo,
F.Martin,
M.Gentile,
A.Santoni,
D.Corda,
G.Poli,
A.Mantovani,
P.Ghezzi,
and
F.Colotta
(2004).
Noncompetitive allosteric inhibitors of the inflammatory chemokine receptors CXCR1 and CXCR2: prevention of reperfusion injury.
|
| |
Proc Natl Acad Sci U S A,
101,
11791-11796.
|
 |
|
|
|
|
 |
W.Liu,
C.E.Rogge,
B.Bambai,
G.Palmer,
A.L.Tsai,
and
R.J.Kulmacz
(2004).
Characterization of the heme environment in Arabidopsis thaliana fatty acid alpha-dioxygenase-1.
|
| |
J Biol Chem,
279,
29805-29815.
|
 |
|
|
|
|
 |
R.J.Kulmacz,
W.A.van der Donk,
and
A.L.Tsai
(2003).
Comparison of the properties of prostaglandin H synthase-1 and -2.
|
| |
Prog Lipid Res,
42,
377-404.
|
 |
|
|
|
|
 |
R.M.Garavito,
and
A.M.Mulichak
(2003).
The structure of mammalian cyclooxygenases.
|
| |
Annu Rev Biophys Biomol Struct,
32,
183-206.
|
 |
|
|
|
|
 |
S.W.Rowlinson,
J.R.Kiefer,
J.J.Prusakiewicz,
J.L.Pawlitz,
K.R.Kozak,
A.S.Kalgutkar,
W.C.Stallings,
R.G.Kurumbail,
and
L.J.Marnett
(2003).
A novel mechanism of cyclooxygenase-2 inhibition involving interactions with Ser-530 and Tyr-385.
|
| |
J Biol Chem,
278,
45763-45769.
|
 |
|
PDB code:
|
 |
|
|
|
|
|
 |
C.Schneider,
W.E.Boeglin,
J.J.Prusakiewicz,
S.W.Rowlinson,
L.J.Marnett,
N.Samel,
and
A.R.Brash
(2002).
Control of prostaglandin stereochemistry at the 15-carbon by cyclooxygenases-1 and -2. A critical role for serine 530 and valine 349.
|
| |
J Biol Chem,
277,
478-485.
|
 |
|
 |
 |
|
The most recent references are shown first.
Citation data come partly from CiteXplore and partly
from an automated harvesting procedure. Note that this is likely to be
only a partial list as not all journals are covered by
either method. However, we are continually building up the citation data
so more and more references will be included with time.
Where a reference describes a PDB structure, the PDB
code is
shown on the right.
|
 |

