 |
PDBsum entry 1g9x

|
|
|

|
 |

|

|

|

|

|

|

|

|

|

|

|

|

|

|

|

|

|

|

|
 |

|

|

|
 |
 |
 |
 |
 |
 |
 |
 |
 |
 |
 |
 |
 |
 |
 |
 |
 |
 |
 |

|

|

|

|

|

|

|

|

|
|
Structural genomics
|
PDB id
|
|

|

|
1g9x
|

|

|

|

|

|

|

|

|

|

|

|

|

|

|

|

|

|

|

|
|
 |
Contents |
 |
|
|

|

|
|
|
|
|
|
|
|
|
|
* Residue conservation analysis
|
|
|
|
|
References listed in PDB file
|
 |
|
Key reference
|
 |
|
Title
|
 |
Structural characterization of an mj1267 ATP-Binding cassette crystal with a complex pattern of twinning caused by promiscuous fiber packing.
|
 |
|
Authors
|
 |
Y.R.Yuan,
O.Martsinkevich,
J.F.Hunt.
|
 |
|
Ref.
|
 |
Acta Crystallogr D Biol Crystallogr, 2003,
59,
225-238.
[DOI no: ]
|
 |
|
PubMed id
|
 |
|
 |
 |
|
Abstract
|
 |
|
ATP-binding cassettes represent the motor domains in ABC transporters, a
superfamily of integral membrane-protein pumps that couple the hydrolysis of ATP
to transmembrane solute translocation. A crystal of a Mg-ADP complex of the
MJ1267 ATP-binding cassette was obtained that produced a diffraction pattern
characterized by pathological streaking of the spots in the a* x b* plane. While
the Laue symmetry of the diffraction pattern was P3;1m, the crystal was
determined to be twinned based on intensity statistics, molecular-replacement
analysis and difference Fourier analysis of an engineered single-site
methylmercury derivative. The unit cell contains three similar 3(1) fibers, with
two of them related by primarily translational non-crystallographic symmetry
(NCS) and the third related to the first two by approximate twofold screw
operations whose rotational components are very similar to the twinning
operator. The promiscuous packing of these 3(1) fibers, which make both parallel
and antiparallel interactions in the primary crystal lattice, can explain the
twinning tendency based on the ability of the twin-related lattices to interact
with one another while making only one slightly sub-optimal intermolecular
contact per unit cell in the boundary region. The promiscuous fiber packing can
also explain the streaking in the diffraction pattern based on the ability to
form a variety of different lattices with similar inter-fiber packing
interactions. The crystal structure was refined as a twin in space group P3(1)
using the program CNS, yielding a free R factor of 28.9% at 2.6 A and a refined
twin fraction of 0.50. The structure shows a rigid-body rotation of the
ABC-transporter-specific alpha-helical subdomain (ABCalpha subdomain) in MJ1267
compared with the conformation observed for the same protein in a C2 crystal
lattice; this observation suggests that the ABCalpha subdomain is flexibly
attached to the F1-type ATP-binding core of the ATP-binding cassette when Mg-ADP
is bound at the active site.
|
 |
 |
 |

|
 |

|
 |
Figure 1.
Figure 1 The trigonal crystal form of MJ1267. (a)
Photomicrograph of typical crystals in approximately 18%(w/v)
PEG 3350, 15%(v/v) ethylene glycol, 10%(v/v) glycerol, 0.1 mM
DTT, 10 mM Mg-ADP, 100 mM MES pH 5.9. (b) A 1° oscillation
frame from a selenomethionine-labeled crystal of the wild-type
protein collected at 12 900 eV on beamline X4A at the NSLS
(displayed with the program XDISP; Otwinowski & Minor,
1997[Otwinowski, Z. & Minor, W. (1997). Methods Enzymol. 276,
307-326.]). (c) A magnified view of the region of the
diffraction image in (a) delineated by the black square.
|
 |
Figure 5.
Figure 5 Stereo pairs showing refined structures of the
Mg-ADP-bound MJ1267 protein. The molecules are color-coded
according to domain organization (Karpowich et al.,
2001[Karpowich, N., Martsinkevich, O., Millen, L., Yuan, Y.-R.,
Dai, P. L., MacVey, K., Thomas, P. J. & Hunt, J. F. (2001).
Structure, 9, 571-586.]), with green showing the antiparallel
 -sheet
subdomain (ABC -sheet
subdomain (ABC  ),
red showing the F1-type ATP-binding core, blue showing the ),
red showing the F1-type ATP-binding core, blue showing the  -helical
subdomain (ABC -helical
subdomain (ABC  )
and magenta showing the )
and magenta showing the 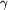 -phosphate
linker which connects the ABC -phosphate
linker which connects the ABC  subdomain
to the ATP-binding core. The images were produced using
MOLSCRIPT and RASTER3D (Kraulis, 1991[Kraulis, P. J. (1991). J.
Appl. Cryst. 24, 946-950.]; Merritt & Bacon, 1997[Merritt, E. A.
& Bacon, D. J. (1997). Methods Enzymol. 277, 505-524.]). (a)
Comparison of the structure of one of the NCS-related molecules
in the twinned trigonal crystal (darker colors) with the
structure of the same protein in the untwinned monoclinic
crystal obtained from the methylmercury derivative of the N109C
mutant of MJ1267 (lighter colors) (Karpowich et al.,
2001[Karpowich, N., Martsinkevich, O., Millen, L., Yuan, Y.-R.,
Dai, P. L., MacVey, K., Thomas, P. J. & Hunt, J. F. (2001).
Structure, 9, 571-586.]). The protein has Mg-ADP bound at the
active site in both crystal structures. The molecules were
aligned based on least-squares superposition of the subdomain
to the ATP-binding core. The images were produced using
MOLSCRIPT and RASTER3D (Kraulis, 1991[Kraulis, P. J. (1991). J.
Appl. Cryst. 24, 946-950.]; Merritt & Bacon, 1997[Merritt, E. A.
& Bacon, D. J. (1997). Methods Enzymol. 277, 505-524.]). (a)
Comparison of the structure of one of the NCS-related molecules
in the twinned trigonal crystal (darker colors) with the
structure of the same protein in the untwinned monoclinic
crystal obtained from the methylmercury derivative of the N109C
mutant of MJ1267 (lighter colors) (Karpowich et al.,
2001[Karpowich, N., Martsinkevich, O., Millen, L., Yuan, Y.-R.,
Dai, P. L., MacVey, K., Thomas, P. J. & Hunt, J. F. (2001).
Structure, 9, 571-586.]). The protein has Mg-ADP bound at the
active site in both crystal structures. The molecules were
aligned based on least-squares superposition of the  -strands
and P-loop helix from the F1-type ATP-binding core. Subunit A
(the `1+' molecule) is shown from the twinned trigonal crystal
structure. (b) Comparison of the three refined NCS-related
molecules in the asymmetric unit of the twinned trigonal crystal
after least-squares superposition of the the -strands
and P-loop helix from the F1-type ATP-binding core. Subunit A
(the `1+' molecule) is shown from the twinned trigonal crystal
structure. (b) Comparison of the three refined NCS-related
molecules in the asymmetric unit of the twinned trigonal crystal
after least-squares superposition of the the  -strands
and P-loop helix from the F1-type ATP-binding core. (c)
Comparison of the three refined NCS-related molecules in the
asymmetric unit of the twinned trigonal crystal after
least-squares superposition of the -strands
and P-loop helix from the F1-type ATP-binding core. (c)
Comparison of the three refined NCS-related molecules in the
asymmetric unit of the twinned trigonal crystal after
least-squares superposition of the  -helices
in the ABC -helices
in the ABC  subdomain. subdomain.
|
 |
|
 |
 |
|
The above figures are
reprinted
by permission from the IUCr:
Acta Crystallogr D Biol Crystallogr
(2003,
59,
225-238)
copyright 2003.
|
 |
|

|
|
|
 |

