
|

|

|
|
 |
Contents |
 |
|
|

|

|

|

|

|

|

|



 655 a.a.
655 a.a.
|
 |

|

|

|

|

|

|

|



 239 a.a.
239 a.a.
|
 |

|

|

|

|

|

|

|



 254 a.a.
254 a.a.
|
 |

|

|
|
|
|
|
|
|
* Residue conservation analysis
|
|
|

|
|
PDB id:
|
 |
|
 |
| Name: |
 |
Oxidoreductase
|
 |
|
Title:
|
 |
Quinol:fumarate reductase from wolinella succinogenes
|
|
Structure:
|
 |
Fumarate reductase flavoprotein subunit. Chain: a, d, g, j. Engineered: yes. Other_details: 8-alpha-[-n-epsilon-histidyl] covalent bond between flavin adenine dinucleotide (fad) and his 43. Fumarate reductase iron-sulfur subunit. Chain: b, e, h, k. Engineered: yes. Fumarate reductase cytochrome b subunit.
|
|
Source:
|
 |
Wolinella succinogenes. Vibrio succinogenes. Organism_taxid: 844. Gene: frda, ws0831. Expressed in: wolinella succinogenes. Expression_system_taxid: 844. Gene: frdb, ws0830. Strain: atcc 29543 / dsm 1740 / lmg 7466 / nctc 11488 / fdc 602w. Variant: frdc-e66q.
|
|
Biol. unit:
|
 |
 Hexamer (from
)
Hexamer (from
)
|
|
Resolution:
|
 |
|
3.10Å
|
R-factor:
|
0.283
|
R-free:
|
0.291
|
|
|
Authors:
|
 |
C.R.D.Lancaster,A.Kroeger
|
Key ref:

|
 |
C.R.Lancaster
et al.
(2001).
A third crystal form of Wolinella succinogenes quinol:fumarate reductase reveals domain closure at the site of fumarate reduction.
Eur J Biochem,
268,
1820-1827.
PubMed id:
DOI:

|
 |
|
Date:
|
 |
|
01-Sep-00
|
Release date:
|
09-Apr-01
|
|
|

|

|
|
PROCHECK
|

|

|
|
|
Headers
|
 |
|
|
References
|
|
|
|

|

|
|
P17412
(FRDA_WOLSU) -
Fumarate reductase flavoprotein subunit from Wolinella succinogenes (strain ATCC 29543 / DSM 1740 / CCUG 13145 / JCM 31913 / LMG 7466 / NCTC 11488 / FDC 602W)
|
|

|

|
Seq:
Struc:
|
 |
 |
 |
656 a.a.
655 a.a.
|

|

|

|

|
|

|

|

|
 |
 |

|
 |
|
|
 |
 |
 |
 |
Enzyme class:
|
 |
Chains A, B, D, E, G, H, J, K:
E.C.1.3.5.1
- succinate dehydrogenase.
|
|
 |
 |
 |
 |
 |

Pathway:
|
 |
Citric acid cycle
|
 |
 |
 |
 |
 |
Reaction:
|
 |
a quinone + succinate = fumarate + a quinol
|
 |
 |
 |
 |
 |
 quinone
quinone
|
+
|
succinate
Bound ligand (Het Group name = )
matches with 50.00% similarity
|
=
|
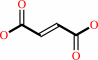 fumarate
fumarate
|
+
|
quinol
|
|
 |
 |
 |
 |
 |
 |
 |
 |
Cofactor:
|
 |
FAD; Iron-sulfur
|
 |
 |
 |
 |
 |
FAD
Bound ligand (Het Group name =
FAD)
corresponds exactly
|
 Iron-sulfur
Iron-sulfur
|
|
 |
 |
 |
 |
|
Molecule diagrams generated from .mol files obtained from the
KEGG ftp site
|
|
 |
 |
 |
 |
 |
 |
 |
 |
 |
 |
 |
 |
 |
 |
 |

|

|

|
| |
|
|
| |
|
DOI no:
|
Eur J Biochem
268:1820-1827
(2001)
|
|
PubMed id:
|
|
|
|

|
| |
|
A third crystal form of Wolinella succinogenes quinol:fumarate reductase reveals domain closure at the site of fumarate reduction.
|
|
C.R.Lancaster,
R.Gross,
J.Simon.
|
|
|

|
| |
ABSTRACT
|
|

|
| |

|
Quinol:fumarate reductase (QFR) is a membrane protein complex that couples the
reduction of fumarate to succinate to the oxidation of quinol to quinone.
Previously, the crystal structure of QFR from Wolinella succinogenes was
determined based on two different crystal forms, and the site of fumarate
binding in the flavoprotein subunit A of the enzyme was located between the
FAD-binding domain and the capping domain [Lancaster, C.R.D., Kröger, A., Auer,
M., & Michel, H. (1999) Nature 402, 377--385]. Here we describe the
structure of W. succinogenes QFR based on a third crystal form and refined at
3.1 A resolution. Compared with the previous crystal forms, the capping domain
is rotated in this structure by approximately 14 degrees relative to the
FAD-binding domain. As a consequence, the topology of the dicarboxylate binding
site is much more similar to those of membrane-bound and soluble fumarate
reductase enzymes from other organisms than to that found in the previous
crystal forms of W. succinogenes QFR. This and the effects of the replacement of
Arg A301 by Glu or Lys by site-directed mutagenesis strongly support a common
mechanism for fumarate reduction in this superfamily of enzymes.

|

|
|
|

|
| |
Selected figure(s)
|
|

|
| |
 |
 |

|
 |

|
 |
Figure 2.
Fig. 2. The site of fumarate reduction in subunit A
(stereo views). The QFR crystal form ‘C’ Arg A301 carbon
atoms are drawn in pink and its nitrogen atoms in light blue.
(A) Electron density maps from the refined model of crystal form
‘C’ at 3.1 Å resolution. Contour levels are 1.0  (2F[o] - F[c,]
blue) and 3.0 (2F[o] - F[c,]
blue) and 3.0  (F[o] - F[c,]
green, with the malonate molecule omitted from the phase
calculation). Due to insufficient density in the 2F[o] - F[c]
map, the side chain of Arg A301 has been assigned zero
occupancy. See text for details. (B) Comparison of W.
succinogenes QFR crystal forms ‘C’ (PDB entry 1E7P, carbon
atoms in yellow, complex with malonate) and ‘B’ (PDB entry
1QLB, carbon atoms in green, complex with fumarate). The
isolated red spheres correspond to the oxygen atoms of two water
molecules in PDB entry 1QLB. (C) Comparison of the crystal
structures of the fumarate reducing sites in W. succinogenes QFR
crystal form ‘C’ (PDB entry 1E7P, carbon atoms in yellow),
in E. coli QFR (PDB entry 1FUM, carbon atoms in white), and in
the S. frigidimarina soluble flavocytochrome c[3] (PDB entry
1QJD, carbon atoms in grey). The different dicarboxylate
compounds included in the models are malonate (1E7P),
oxaloacetate (1FUM), and a malate-like intermediate (1QJD).
Residues are numbered according to W. succinogenes QFR. (F[o] - F[c,]
green, with the malonate molecule omitted from the phase
calculation). Due to insufficient density in the 2F[o] - F[c]
map, the side chain of Arg A301 has been assigned zero
occupancy. See text for details. (B) Comparison of W.
succinogenes QFR crystal forms ‘C’ (PDB entry 1E7P, carbon
atoms in yellow, complex with malonate) and ‘B’ (PDB entry
1QLB, carbon atoms in green, complex with fumarate). The
isolated red spheres correspond to the oxygen atoms of two water
molecules in PDB entry 1QLB. (C) Comparison of the crystal
structures of the fumarate reducing sites in W. succinogenes QFR
crystal form ‘C’ (PDB entry 1E7P, carbon atoms in yellow),
in E. coli QFR (PDB entry 1FUM, carbon atoms in white), and in
the S. frigidimarina soluble flavocytochrome c[3] (PDB entry
1QJD, carbon atoms in grey). The different dicarboxylate
compounds included in the models are malonate (1E7P),
oxaloacetate (1FUM), and a malate-like intermediate (1QJD).
Residues are numbered according to W. succinogenes QFR.
|
 |
Figure 3.
Fig. 3. Possible mechanism of fumarate reduction in W.
succinogenes QFR involving the residues shown in Fig. 2 Go- . Hydride
transfer from the N5 of FAD to the . Hydride
transfer from the N5 of FAD to the  -methenyl of
fumarate (in blue) is coupled to proton transfer to the -methenyl of
fumarate (in blue) is coupled to proton transfer to the  position of
the substrate from the side chain of Arg A301. position of
the substrate from the side chain of Arg A301.
|
 |
|
|

|
| |
The above figures are
reprinted
by permission from the Federation of European Biochemical Societies:
Eur J Biochem
(2001,
268,
1820-1827)
copyright 2001.
|
|
| |
Figures were
selected
by an automated process.
|
|

|

|
|
 |
 |
|
 |
 |
 |
 |
 |
 |
 |
 |
 |
|
Literature references that cite this PDB file's key reference
|
|
 |
| |
PubMed id
|
 |
Reference
|
 |
|
|
|
 |
H.D.Juhnke,
H.Hiltscher,
H.R.Nasiri,
H.Schwalbe,
and
C.R.Lancaster
(2009).
Production, characterization and determination of the real catalytic properties of the putative 'succinate dehydrogenase' from Wolinella succinogenes.
|
| |
Mol Microbiol,
71,
1088-1101.
|
 |
|
|
|
|
 |
T.M.Tomasiak,
E.Maklashina,
G.Cecchini,
and
T.M.Iverson
(2008).
A threonine on the active site loop controls transition state formation in Escherichia coli respiratory complex II.
|
| |
J Biol Chem,
283,
15460-15468.
|
 |
|
PDB code:
|
 |
|
|
|
|
|
 |
J.D.Otero-Cruz,
C.A.Báez-Pagán,
I.M.Caraballo-González,
and
J.A.Lasalde-Dominicci
(2007).
Tryptophan-scanning mutagenesis in the alphaM3 transmembrane domain of the muscle-type acetylcholine receptor. A spring model revealed.
|
| |
J Biol Chem,
282,
9162-9171.
|
 |
|
|
|
|
 |
K.L.Pankhurst,
C.G.Mowat,
E.L.Rothery,
J.M.Hudson,
A.K.Jones,
C.S.Miles,
M.D.Walkinshaw,
F.A.Armstrong,
G.A.Reid,
and
S.K.Chapman
(2006).
A proton delivery pathway in the soluble fumarate reductase from Shewanella frigidimarina.
|
| |
J Biol Chem,
281,
20589-20597.
|
 |
|
PDB codes:
|
 |
|
|
|
|
|
 |
L.S.Huang,
G.Sun,
D.Cobessi,
A.C.Wang,
J.T.Shen,
E.Y.Tung,
V.E.Anderson,
and
E.A.Berry
(2006).
3-nitropropionic acid is a suicide inhibitor of mitochondrial respiration that, upon oxidation by complex II, forms a covalent adduct with a catalytic base arginine in the active site of the enzyme.
|
| |
J Biol Chem,
281,
5965-5972.
|
 |
|
PDB codes:
|
 |
|
|
|
|
|
 |
L.S.Huang,
J.T.Shen,
A.C.Wang,
and
E.A.Berry
(2006).
Crystallographic studies of the binding of ligands to the dicarboxylate site of Complex II, and the identity of the ligand in the "oxaloacetate-inhibited" state.
|
| |
Biochim Biophys Acta,
1757,
1073-1083.
|
 |
|
PDB codes:
|
 |
|
|
|
|
|
 |
M.G.Madej,
H.R.Nasiri,
N.S.Hilgendorff,
H.Schwalbe,
and
C.R.Lancaster
(2006).
Evidence for transmembrane proton transfer in a dihaem-containing membrane protein complex.
|
| |
EMBO J,
25,
4963-4970.
|
 |
|
PDB code:
|
 |
|
|
|
|
|
 |
C.R.Lancaster,
U.S.Sauer,
R.Gross,
A.H.Haas,
J.Graf,
H.Schwalbe,
W.Mäntele,
J.Simon,
and
M.G.Madej
(2005).
Experimental support for the "E pathway hypothesis" of coupled transmembrane e- and H+ transfer in dihemic quinol:fumarate reductase.
|
| |
Proc Natl Acad Sci U S A,
102,
18860-18865.
|
 |
|
PDB codes:
|
 |
|
|
|
|
|
 |
H.Kneuper,
I.G.Janausch,
V.Vijayan,
M.Zweckstetter,
V.Bock,
C.Griesinger,
and
G.Unden
(2005).
The nature of the stimulus and of the fumarate binding site of the fumarate sensor DcuS of Escherichia coli.
|
| |
J Biol Chem,
280,
20596-20603.
|
 |
|
|
|
|
 |
A.H.Haas,
and
C.R.Lancaster
(2004).
Calculated coupling of transmembrane electron and proton transfer in dihemic quinol:fumarate reductase.
|
| |
Biophys J,
87,
4298-4315.
|
 |
|
|
|
|
 |
J.Guo,
and
B.D.Lemire
(2003).
The ubiquinone-binding site of the Saccharomyces cerevisiae succinate-ubiquinone oxidoreductase is a source of superoxide.
|
| |
J Biol Chem,
278,
47629-47635.
|
 |
|
|
|
|
 |
P.A.Hubbard,
X.Liang,
H.Schulz,
and
J.J.Kim
(2003).
The crystal structure and reaction mechanism of Escherichia coli 2,4-dienoyl-CoA reductase.
|
| |
J Biol Chem,
278,
37553-37560.
|
 |
|
PDB code:
|
 |
|
|
|
|
|
 |
J.Simon
(2002).
Enzymology and bioenergetics of respiratory nitrite ammonification.
|
| |
FEMS Microbiol Rev,
26,
285-309.
|
 |
|
|
|
|
 |
R.T.Bossi,
A.Negri,
G.Tedeschi,
and
A.Mattevi
(2002).
Structure of FAD-bound L-aspartate oxidase: insight into substrate specificity and catalysis.
|
| |
Biochemistry,
41,
3018-3024.
|
 |
|
PDB codes:
|
 |
|
|
|
|
|
 |
S.J.Charnock,
I.E.Brown,
J.P.Turkenburg,
G.W.Black,
and
G.J.Davies
(2002).
Convergent evolution sheds light on the anti-beta -elimination mechanism common to family 1 and 10 polysaccharide lyases.
|
| |
Proc Natl Acad Sci U S A,
99,
12067-12072.
|
 |
|
PDB codes:
|
 |
|
|
 |
 |
|
The most recent references are shown first.
Citation data come partly from CiteXplore and partly
from an automated harvesting procedure. Note that this is likely to be
only a partial list as not all journals are covered by
either method. However, we are continually building up the citation data
so more and more references will be included with time.
Where a reference describes a PDB structure, the PDB
code is
shown on the right.
|
 |
|

