 |
PDBsum entry 1qjd

|
|
|

|
 |

|

|

|

|

|

|

|

|

|

|

|

|

|

|
 |

|

|

|
 |
 |
 |
 |
 |
 |
 |
 |
 |
 |
 |
 |
 |
 |
 |
 |
 |
 |

|

|

|

|

|

|

|

|

|
|
Oxidoreductase
|
PDB id
|
|

|

|
1qjd
|

|

|

|

|

|

|

|

|

|

|

|

|

|

|

|

|

|

|

|
|
 |
Contents |
 |
|
|

|

|
|
|
|
|
|
|
|
|
|
* Residue conservation analysis
|
|
|
|
 |
|
|
 |
 |
 |
 |
Enzyme class:
|
 |
E.C.1.3.2.4
- fumarate reductase (cytochrome).
|
|
 |
 |
 |
 |
 |
Reaction:
|
 |
2 Fe(III)-[cytochrome c] + succinate = fumarate + 2 Fe(II)-[cytochrome c] + 2 H+
|
 |
 |
 |
 |
 |
2
×
Fe(III)-[cytochrome c]
|
+
|
succinate
Bound ligand (Het Group name = )
matches with 88.89% similarity
|
=
|
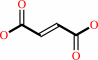 fumarate
fumarate
|
+
|
2
×
Fe(II)-[cytochrome c]
|
+
|
2
×
H(+)
|
|
 |
 |
 |
 |
 |
 |
 |
 |
 |
 |
|
Molecule diagrams generated from .mol files obtained from the
KEGG ftp site
|
|
 |
 |
 |
 |
 |
 |
 |
 |
 |
 |
 |
 |
 |
 |
 |

|

|

|
| |
|
|
| |
|
DOI no:
|
Nat Struct Biol
6:1108-1112
(1999)
|
|
PubMed id:
|
|
|
|

|
| |
|
Structural and mechanistic mapping of a unique fumarate reductase.
|
|
P.Taylor,
S.L.Pealing,
G.A.Reid,
S.K.Chapman,
M.D.Walkinshaw.
|
|
|

|
| |
ABSTRACT
|
|

|
| |

|
The 1.8 A resolution crystal structure of the tetraheme flavocytochrome c3,
Fcc3, provides the first mechanistic insight into respiratory fumarate
reductases or succinate dehydrogenases. The multi-redox center, three-domain
protein shows a 40 A long 'molecular wire' allowing rapid conduction of
electrons through a new type of cytochrome domain onto the active site flavin,
driving the reduction of fumarate to succinate. In this structure a malate-like
molecule is trapped in the enzyme active site. The interactions between this
molecule and the enzyme suggest a clear mechanism for fumarate reduction in
which the substrate is polarized and twisted, facilitating hydride transfer from
the reduced flavin and subsequent proton transfer. The enzyme active site in the
oxidized form is completely buried at the interface between the flavin-binding
and the clamp domains. Movement of the cytochrome and clamp domains is
postulated to allow release of the product.

|

|
|
|

|
| |
Selected figure(s)
|
|

|
| |
 |
 |

|
 |

|
 |
Figure 3.
Figure 3. The active site of Fcc[3]. a, Stereo MOLSCRIPT^30
representation of the environment of the modified substrate.
Hydrogen bonds are shown as red dashed lines. Other short
contacts are shown as green dashed lines. Distances are also
tabulated showing hydrogen bonds and short nonbonded contacts
(<3.1 Å) involving the modified substrate. b, Connolly
surfaces of the available active site volume (green) and
substrate-occupied volume (red). The Connolly surface of the
available volume in the substrate binding site (transparent
green surface) is almost completely filled by the substrate
intermediate molecule. The Connolly surface of the substrate
intermediate molecule shown as a wire representation colored
red. c, An overlay of a modeled planar fumarate (magenta) and
the modified substrate. Short contacts between the modeled
fumarate molecule and the two clamping methionine residues are
shown. The resulting twist in the conformation of the carboxyl
group is important in the enzyme mechanism.
|
 |
Figure 4.
Figure 4. Reaction mechanisms at the Fcc[3] active site. a,
Schematic representation of the mechanism of fumarate reduction
by Fcc[3.] Catalysis is initiated by the binding of fumarate at
the active site. The C1 carboxylate group of fumarate (to the
left in this representation) is twisted out of plane by the
closure of the clamp domain and the resulting steric constraints
imposed by the side chains of Met 236 and Met 375 (Fig. 3d) and
by hydrogen bonding to His 365. The substrate C4 carboxylate is
bound in a very positively charged environment involving
interactions with His 504, Arg 544 and Arg 402. The combination
of these effects results in polarization of the C2−C3 bond
with the build up of positive charge at C2, facilitating hydride
transfer from N5 of the reduced flavin to the si-face of the
substrate. Arg 402 which is only 2.99 Å from C3, is
ideally positioned for proton transfer, resulting in the
formation of the product, succinate. b, Proposed mechanism for
the formation of the hydrated intermediate at the active site of
the oxidized enzyme. In the oxidized flavocytochrome c[3], as is
the case in the crystal, there is no hydride available to attack
the substrate C2 atom. Instead, water acts as a nucleophile
attacking the re-face. The resulting intermediate, which is
shown in the electron density to have R-stereochemistry at C2,
is trapped at the active site in the crystal. c, Conservation of
active site residues in fumarate reductases and succinate
dehydrogenases. Segments of the sequence of Fcc[3] are aligned
with the corresponding regions of the flavoprotein subunits of
the fumarate reductases from E. coli (frda_ecoli) and Wolinella
succinogenes (frda_wolsu) and the succinate dehydrogenases from
E.coli (dhsa_ecoli) and Saccharomyces cerevisiae (dhsa_yeast) to
highlight the conservation around active site residues. The
following residues are highlighted in red: His 365 and Thr 377
(top left), Arg 402 (top right), His 504 (bottom left) and Arg
544 (bottom right).
|
 |
|
|

|
| |
The above figures are
reprinted
by permission from Macmillan Publishers Ltd:
Nat Struct Biol
(1999,
6,
1108-1112)
copyright 1999.
|
|
| |
Figures were
selected
by an automated process.
|
|

|

|
|
 |
 |
|
 |
 |
 |
 |
 |
 |
 |
 |
 |
|
Literature references that cite this PDB file's key reference
|
|
 |
| |
PubMed id
|
 |
Reference
|
 |
|
|
|
 |
C.Lockwood,
J.N.Butt,
T.A.Clarke,
and
D.J.Richardson
(2011).
Molecular interactions between multihaem cytochromes: probing the protein-protein interactions between pentahaem cytochromes of a nitrite reductase complex.
|
| |
Biochem Soc Trans,
39,
263-268.
|
 |
|
|
|
|
 |
C.M.Paquete,
and
R.O.Louro
(2010).
Molecular details of multielectron transfer: the case of multiheme cytochromes from metal respiring organisms.
|
| |
Dalton Trans,
39,
4259-4266.
|
 |
|
|
|
|
 |
H.D.Juhnke,
H.Hiltscher,
H.R.Nasiri,
H.Schwalbe,
and
C.R.Lancaster
(2009).
Production, characterization and determination of the real catalytic properties of the putative 'succinate dehydrogenase' from Wolinella succinogenes.
|
| |
Mol Microbiol,
71,
1088-1101.
|
 |
|
|
|
|
 |
J.Ruprecht,
V.Yankovskaya,
E.Maklashina,
S.Iwata,
and
G.Cecchini
(2009).
Structure of Escherichia coli succinate:quinone oxidoreductase with an occupied and empty quinone-binding site.
|
| |
J Biol Chem,
284,
29836-29846.
|
 |
|
PDB codes:
|
 |
|
|
|
|
|
 |
J.I.Yeh,
U.Chinte,
and
S.Du
(2008).
Structure of glycerol-3-phosphate dehydrogenase, an essential monotopic membrane enzyme involved in respiration and metabolism.
|
| |
Proc Natl Acad Sci U S A,
105,
3280-3285.
|
 |
|
PDB codes:
|
 |
|
|
|
|
|
 |
T.M.Tomasiak,
E.Maklashina,
G.Cecchini,
and
T.M.Iverson
(2008).
A threonine on the active site loop controls transition state formation in Escherichia coli respiratory complex II.
|
| |
J Biol Chem,
283,
15460-15468.
|
 |
|
PDB code:
|
 |
|
|
|
|
|
 |
A.K.Upadhyay,
A.B.Hooper,
and
M.P.Hendrich
(2006).
NO reductase activity of the tetraheme cytochrome C554 of Nitrosomonas europaea.
|
| |
J Am Chem Soc,
128,
4330-4337.
|
 |
|
|
|
|
 |
E.Maklashina,
T.M.Iverson,
Y.Sher,
V.Kotlyar,
J.Andréll,
O.Mirza,
J.M.Hudson,
F.A.Armstrong,
R.A.Rothery,
J.H.Weiner,
and
G.Cecchini
(2006).
Fumarate reductase and succinate oxidase activity of Escherichia coli complex II homologs are perturbed differently by mutation of the flavin binding domain.
|
| |
J Biol Chem,
281,
11357-11365.
|
 |
|
PDB code:
|
 |
|
|
|
|
|
 |
J.E.Butler,
R.H.Glaven,
A.Esteve-Núñez,
C.Núñez,
E.S.Shelobolina,
D.R.Bond,
and
D.R.Lovley
(2006).
Genetic characterization of a single bifunctional enzyme for fumarate reduction and succinate oxidation in Geobacter sulfurreducens and engineering of fumarate reduction in Geobacter metallireducens.
|
| |
J Bacteriol,
188,
450-455.
|
 |
|
|
|
|
 |
J.Zhang,
F.E.Frerman,
and
J.J.Kim
(2006).
Structure of electron transfer flavoprotein-ubiquinone oxidoreductase and electron transfer to the mitochondrial ubiquinone pool.
|
| |
Proc Natl Acad Sci U S A,
103,
16212-16217.
|
 |
|
PDB codes:
|
 |
|
|
|
|
|
 |
K.L.Pankhurst,
C.G.Mowat,
E.L.Rothery,
J.M.Hudson,
A.K.Jones,
C.S.Miles,
M.D.Walkinshaw,
F.A.Armstrong,
G.A.Reid,
and
S.K.Chapman
(2006).
A proton delivery pathway in the soluble fumarate reductase from Shewanella frigidimarina.
|
| |
J Biol Chem,
281,
20589-20597.
|
 |
|
PDB codes:
|
 |
|
|
|
|
|
 |
L.S.Huang,
G.Sun,
D.Cobessi,
A.C.Wang,
J.T.Shen,
E.Y.Tung,
V.E.Anderson,
and
E.A.Berry
(2006).
3-nitropropionic acid is a suicide inhibitor of mitochondrial respiration that, upon oxidation by complex II, forms a covalent adduct with a catalytic base arginine in the active site of the enzyme.
|
| |
J Biol Chem,
281,
5965-5972.
|
 |
|
PDB codes:
|
 |
|
|
|
|
|
 |
L.S.Huang,
J.T.Shen,
A.C.Wang,
and
E.A.Berry
(2006).
Crystallographic studies of the binding of ligands to the dicarboxylate site of Complex II, and the identity of the ligand in the "oxaloacetate-inhibited" state.
|
| |
Biochim Biophys Acta,
1757,
1073-1083.
|
 |
|
PDB codes:
|
 |
|
|
|
|
|
 |
C.G.Mowat,
and
S.K.Chapman
(2005).
Multi-heme cytochromes--new structures, new chemistry.
|
| |
Dalton Trans,
(),
3381-3389.
|
 |
|
|
|
|
 |
A.Crow,
R.M.Acheson,
N.E.Le Brun,
and
A.Oubrie
(2004).
Structural basis of Redox-coupled protein substrate selection by the cytochrome c biosynthesis protein ResA.
|
| |
J Biol Chem,
279,
23654-23660.
|
 |
|
PDB codes:
|
 |
|
|
|
|
|
 |
T.A.Clarke,
V.Dennison,
H.E.Seward,
B.Burlat,
J.A.Cole,
A.M.Hemmings,
and
D.J.Richardson
(2004).
Purification and spectropotentiometric characterization of Escherichia coli NrfB, a decaheme homodimer that transfers electrons to the decaheme periplasmic nitrite reductase complex.
|
| |
J Biol Chem,
279,
41333-41339.
|
 |
|
|
|
|
 |
T.E.Meyer,
A.I.Tsapin,
I.Vandenberghe,
L.de Smet,
D.Frishman,
K.H.Nealson,
M.A.Cusanovich,
and
J.J.van Beeumen
(2004).
Identification of 42 possible cytochrome C genes in the Shewanella oneidensis genome and characterization of six soluble cytochromes.
|
| |
OMICS,
8,
57-77.
|
 |
|
|
|
|
 |
A.C.Price,
C.O.Rock,
and
S.W.White
(2003).
The 1.3-Angstrom-resolution crystal structure of beta-ketoacyl-acyl carrier protein synthase II from Streptococcus pneumoniae.
|
| |
J Bacteriol,
185,
4136-4143.
|
 |
|
PDB codes:
|
 |
|
|
|
|
|
 |
F.Reyes-Ramirez,
P.Dobbin,
G.Sawers,
and
D.J.Richardson
(2003).
Characterization of transcriptional regulation of Shewanella frigidimarina Fe(III)-induced flavocytochrome c reveals a novel iron-responsive gene regulation system.
|
| |
J Bacteriol,
185,
4564-4571.
|
 |
|
|
|
|
 |
G.Cecchini
(2003).
Function and structure of complex II of the respiratory chain.
|
| |
Annu Rev Biochem,
72,
77.
|
 |
|
|
|
|
 |
K.E.Pitts,
P.S.Dobbin,
F.Reyes-Ramirez,
A.J.Thomson,
D.J.Richardson,
and
H.E.Seward
(2003).
Characterization of the Shewanella oneidensis MR-1 decaheme cytochrome MtrA: expression in Escherichia coli confers the ability to reduce soluble Fe(III) chelates.
|
| |
J Biol Chem,
278,
27758-27765.
|
 |
|
|
|
|
 |
A.Brigé,
D.Leys,
T.E.Meyer,
M.A.Cusanovich,
and
J.J.Van Beeumen
(2002).
The 1.25 A resolution structure of the diheme NapB subunit of soluble nitrate reductase reveals a novel cytochrome c fold with a stacked heme arrangement.
|
| |
Biochemistry,
41,
4827-4836.
|
 |
|
PDB code:
|
 |
|
|
|
|
|
 |
A.W.Munro,
D.G.Leys,
K.J.McLean,
K.R.Marshall,
T.W.Ost,
S.Daff,
C.S.Miles,
S.K.Chapman,
D.A.Lysek,
C.C.Moser,
C.C.Page,
and
P.L.Dutton
(2002).
P450 BM3: the very model of a modern flavocytochrome.
|
| |
Trends Biochem Sci,
27,
250-257.
|
 |
|
|
|
|
 |
C.A.Bottoms,
P.E.Smith,
and
J.J.Tanner
(2002).
A structurally conserved water molecule in Rossmann dinucleotide-binding domains.
|
| |
Protein Sci,
11,
2125-2137.
|
 |
|
|
|
|
 |
D.Leys,
T.E.Meyer,
A.S.Tsapin,
K.H.Nealson,
M.A.Cusanovich,
and
J.J.Van Beeumen
(2002).
Crystal structures at atomic resolution reveal the novel concept of "electron-harvesting" as a role for the small tetraheme cytochrome c.
|
| |
J Biol Chem,
277,
35703-35711.
|
 |
|
PDB codes:
|
 |
|
|
|
|
|
 |
I.J.Correia,
C.M.Paquete,
R.O.Louro,
T.Catarino,
D.L.Turner,
and
A.V.Xavier
(2002).
Thermodynamic and kinetic characterization of trihaem cytochrome c3 from Desulfuromonas acetoxidans.
|
| |
Eur J Biochem,
269,
5722-5730.
|
 |
|
|
|
|
 |
R.T.Bossi,
A.Negri,
G.Tedeschi,
and
A.Mattevi
(2002).
Structure of FAD-bound L-aspartate oxidase: insight into substrate specificity and catalysis.
|
| |
Biochemistry,
41,
3018-3024.
|
 |
|
PDB codes:
|
 |
|
|
|
|
|
 |
V.A.Bamford,
H.C.Angove,
H.E.Seward,
A.J.Thomson,
J.A.Cole,
J.N.Butt,
A.M.Hemmings,
and
D.J.Richardson
(2002).
Structure and spectroscopy of the periplasmic cytochrome c nitrite reductase from Escherichia coli.
|
| |
Biochemistry,
41,
2921-2931.
|
 |
|
PDB code:
|
 |
|
|
|
|
|
 |
A.I.Tsapin,
I.Vandenberghe,
K.H.Nealson,
J.H.Scott,
T.E.Meyer,
M.A.Cusanovich,
E.Harada,
T.Kaizu,
H.Akutsu,
D.Leys,
and
J.J.Van Beeumen
(2001).
Identification of a small tetraheme cytochrome c and a flavocytochrome c as two of the principal soluble cytochromes c in Shewanella oneidensis strain MR1.
|
| |
Appl Environ Microbiol,
67,
3236-3244.
|
 |
|
|
|
|
 |
C.R.Lancaster,
R.Gross,
and
J.Simon
(2001).
A third crystal form of Wolinella succinogenes quinol:fumarate reductase reveals domain closure at the site of fumarate reduction.
|
| |
Eur J Biochem,
268,
1820-1827.
|
 |
|
PDB code:
|
 |
|
|
|
|
|
 |
G.Tedeschi,
S.Ronchi,
T.Simonic,
C.Treu,
A.Mattevi,
and
A.Negri
(2001).
Probing the active site of L-aspartate oxidase by site-directed mutagenesis: role of basic residues in fumarate reduction.
|
| |
Biochemistry,
40,
4738-4744.
|
 |
|
|
|
|
 |
A.W.Munro,
P.Taylor,
and
M.D.Walkinshaw
(2000).
Structures of redox enzymes.
|
| |
Curr Opin Biotechnol,
11,
369-376.
|
 |
|
|
|
|
 |
C.R.Lancaster,
and
A.Kröger
(2000).
Succinate: quinone oxidoreductases: new insights from X-ray crystal structures.
|
| |
Biochim Biophys Acta,
1459,
422-431.
|
 |
|
|
|
|
 |
G.A.Reid,
C.S.Miles,
R.K.Moysey,
K.L.Pankhurst,
and
S.K.Chapman
(2000).
Catalysis in fumarate reductase.
|
| |
Biochim Biophys Acta,
1459,
310-315.
|
 |
|
|
|
|
 |
T.M.Iverson,
C.Luna-Chavez,
I.Schröder,
G.Cecchini,
and
D.C.Rees
(2000).
Analyzing your complexes: structure of the quinol-fumarate reductase respiratory complex.
|
| |
Curr Opin Struct Biol,
10,
448-455.
|
 |
|
|
|
|
 |
P.D.Barker,
and
S.J.Ferguson
(1999).
Still a puzzle: why is haem covalently attached in c-type cytochromes?
|
| |
Structure,
7,
R281-R290.
|
 |
|
 |
 |
|
The most recent references are shown first.
Citation data come partly from CiteXplore and partly
from an automated harvesting procedure. Note that this is likely to be
only a partial list as not all journals are covered by
either method. However, we are continually building up the citation data
so more and more references will be included with time.
Where a reference describes a PDB structure, the PDB
codes are
shown on the right.
|
 |

