 |
PDBsum entry 6ut0

|
|
|

|
 |

|

|

|

|

|

|

|

|

|

|

|

|

|

|

|

|

|

|

|

|
 |

|

|

|
 |
 |
 |
 |
 |
 |
 |
 |
 |
 |
 |
 |
 |
 |
 |
 |
 |
 |
 |
 |
 |

|

|

|

|

|

|

|

|

|
|
Hydrolase/inhibitor
|
PDB id
|
|

|

|
6ut0
|

|

|

|

|

|

|

|

|

|

|

|

|

|

|

|

|
|
|

|
|
PDB id:
|
 |
|
 |
| Name: |
 |
Hydrolase/inhibitor
|
 |
|
Title:
|
 |
Identification of the clinical development candidate mrtx849, a covalent krasg12c inhibitor for the treatment of cancer
|
|
Structure:
|
 |
Gtpase kras. Chain: a, b, c, d. Synonym: k-ras 2,ki-ras,c-k-ras,c-ki-ras. Engineered: yes. Mutation: yes
|
|
Source:
|
 |
Homo sapiens. Human. Organism_taxid: 9606. Gene: kras, kras2, rask2. Expressed in: escherichia coli. Expression_system_taxid: 562
|
|
Resolution:
|
 |
|
1.94Å
|
R-factor:
|
0.175
|
R-free:
|
0.222
|
|
|
Authors:
|
 |
G.P.Vigers,D.J.Smith
|
|
Key ref:
|
 |
J.B.Fell
et al.
(2020).
Identification of the Clinical Development Candidate MRTX849, a Covalent KRASG12C Inhibitor for the Treatment of Cancer.
J Med Chem,
63,
6679-6693.
PubMed id:
DOI:
|
 |
|
Date:
|
 |
|
29-Oct-19
|
Release date:
|
22-Apr-20
|
|
|

|

|
|
PROCHECK
|

|

|
|
|
Headers
|
 |
|
|
References
|
|
|
|

|

|
|
P01116
(RASK_HUMAN) -
GTPase KRas from Homo sapiens
|
|

|

|
Seq:
Struc:
|
 |
 |
 |
189 a.a.
169 a.a.*
|

|

|

|

|
|

|

|

|
 |
 |

|
|
Key: |
 |
PfamA domain |
 |
 |
 |
Secondary structure |
 |
|
*
PDB and UniProt seqs differ
at 10 residue positions (black
crosses)
|
|

|
|
|
 |
|
|
 |
 |
 |
 |
Enzyme class:
|
 |
E.C.3.6.5.2
- small monomeric GTPase.
|
|
 |
 |
 |
 |
 |
Reaction:
|
 |
GTP + H2O = GDP + phosphate + H+
|
 |
 |
 |
 |
 |
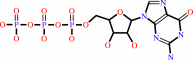 GTP
GTP
|
+
|
H2O
|
=
|
GDP
Bound ligand (Het Group name = )
corresponds exactly
|
+
|
 phosphate
phosphate
|
+
|
H(+)
|
|
 |
 |
 |
 |
 |
 |
 |
 |
 |
 |
|
Molecule diagrams generated from .mol files obtained from the
KEGG ftp site
|
|
 |
 |
 |
 |
 |
 |
 |
 |
 |
 |
 |
 |
 |
 |
 |

|

|

|
| |
|
|
| |
|
DOI no:
|
J Med Chem
63:6679-6693
(2020)
|
|
PubMed id:
|
|
|
|

|
| |
|
Identification of the Clinical Development Candidate MRTX849, a Covalent KRASG12C Inhibitor for the Treatment of Cancer.
|
|
J.B.Fell,
J.P.Fischer,
B.R.Baer,
J.F.Blake,
K.Bouhana,
D.M.Briere,
K.D.Brown,
L.E.Burgess,
A.C.Burns,
M.R.Burkard,
H.Chiang,
M.J.Chicarelli,
A.W.Cook,
J.J.Gaudino,
J.Hallin,
L.Hanson,
D.P.Hartley,
E.J.Hicken,
G.P.Hingorani,
R.J.Hinklin,
M.J.Mejia,
P.Olson,
J.N.Otten,
S.P.Rhodes,
M.E.Rodriguez,
P.Savechenkov,
D.J.Smith,
N.Sudhakar,
F.X.Sullivan,
T.P.Tang,
G.P.Vigers,
L.Wollenberg,
J.G.Christensen,
M.A.Marx.
|
|
|

|
| |
ABSTRACT
|
|

|
| |

|
Capping off an era marred by drug development failures and punctuated by waning
interest and presumed intractability toward direct targeting of KRAS, new
technologies and strategies are aiding in the target's resurgence. As previously
reported, the tetrahydropyridopyrimidines were identified as irreversible
covalent inhibitors of KRASG12C that bind in the switch-II pocket of
KRAS and make a covalent bond to cysteine 12. Using structure-based drug design
in conjunction with a focused in vitro absorption, distribution, metabolism and
excretion screening approach, analogues were synthesized to increase the potency
and reduce metabolic liabilities of this series. The discovery of the clinical
development candidate MRTX849 as a potent, selective covalent inhibitor
of KRASG12C is described.

|

|
|
|

|

|
|
 |
 |
|
 |
 |
 |
 |
 |
 |
 |
 |
 |
 |

