 |
PDBsum entry 6fd6
 |
|
|
 |
 |
 |
 |
Enzyme class:
|
 |
E.C.2.4.2.7
- adenine phosphoribosyltransferase.
|
|
 |
 |
 |
 |
 |

Pathway:
|
 |
Ribose activation
|
 |
 |
 |
 |
 |
Reaction:
|
 |
AMP + diphosphate = 5-phospho-alpha-D-ribose 1-diphosphate + adenine
|
 |
 |
 |
 |
 |
AMP
Bound ligand (Het Group name = )
corresponds exactly
|
+
|
diphosphate
Bound ligand (Het Group name = )
corresponds exactly
|
=
|
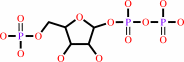 5-phospho-alpha-D-ribose 1-diphosphate
5-phospho-alpha-D-ribose 1-diphosphate
|
+
|
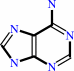 adenine
adenine
|
|
 |
 |
 |
 |
 |
 |
 |
 |
 |
 |
|
Molecule diagrams generated from .mol files obtained from the
KEGG ftp site
|
|
 |
 |
 |
 |
 |
 |
 |
 |
 |
 |
 |
 |
 |
 |
 |

|

|

|
| |
|
|
| |
|
DOI no:
|
Cell Chem Biol
25:666
(2018)
|
|
PubMed id:
|
|
|
|

|
| |
|
Structural Insights into the Forward and Reverse Enzymatic Reactions in Human Adenine Phosphoribosyltransferase.
|
|
J.Huyet,
M.Ozeir,
M.C.Burgevin,
B.Pinson,
F.Chesney,
J.M.Remy,
A.R.Siddiqi,
R.Lupoli,
G.Pinon,
C.Saint-Marc,
J.F.Gibert,
R.Morales,
I.Ceballos-Picot,
R.Barouki,
B.Daignan-Fornier,
A.Olivier-Bandini,
F.Augé,
P.Nioche.
|
|
|

|
| |
ABSTRACT
|
|

|
| |

|
Phosphoribosyltransferases catalyze the displacement of a PRPP
α-1'-pyrophosphate to a nitrogen-containing nucleobase. How they control the
balance of substrates/products binding and activities is poorly understood.
Here, we investigated the human adenine phosphoribosyltransferase (hAPRT) that
produces AMP in the purine salvage pathway. We show that a single oxygen atom
from the Tyr105 side chain is responsible for selecting the active conformation
of the 12 amino acid long catalytic loop. Using in vitro, cellular, and in
crystallo approaches, we demonstrated that Tyr105 is key for the fine-tuning of
the kinetic activity efficiencies of the forward and reverse reactions.
Together, our results reveal an evolutionary pressure on the strictly conserved
Tyr105 and on the dynamic motion of the flexible loop in
phosphoribosyltransferases that is essential for purine biosynthesis in cells.
These data also provide the framework for designing novel adenine derivatives
that could modulate, through hAPRT, diseases-involved cellular pathways.

|

|
|
|

|

|
|
 |
 |
|
 |
 |
 |
 |
 |
 |
 |
 |
 |
 |

