 |
PDBsum entry 5ufe

|
|
|

|
 |

|

|

|

|

|

|

|

|

|

|

|

|

|

|

|

|

|

|
 |

|

|

|
 |
 |
 |
 |
 |
 |
 |
 |
 |
 |
 |
 |
 |
 |
 |
 |
 |
 |
 |
 |

|

|

|

|

|

|

|

|

|
|
Hydrolase/de novo protein
|
PDB id
|
|

|

|
5ufe
|

|

|

|

|

|

|

|

|

|

|

|

|

|

|

|

|

|

|

|
|
 |
Contents |
 |
|
|

|

|
|
|
|
|

|

|

|

|

|
 _CO
×3 _CO
×3
|
 |

|

|

|

|

|

|

|
 _CA
×3 _CA
×3
|
 |

|

|

|

|

|

|

|
 _CD
×4 _CD
×4
|
 |

|

|

|

|

|

|

|
 _CL
×3 _CL
×3
|
 |

|

|

|

|

|

|

|
 _MG _MG
|
 |

|

|
|
|
|
|
|
 |
|
|
 |
 |
 |
 |
Enzyme class:
|
 |
Chain A:
E.C.3.6.5.2
- small monomeric GTPase.
|
|
 |
 |
 |
 |
 |
Reaction:
|
 |
GTP + H2O = GDP + phosphate + H+
|
 |
 |
 |
 |
 |
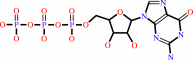 GTP
GTP
|
+
|
H2O
|
=
|
GDP
Bound ligand (Het Group name = )
matches with 81.82% similarity
|
+
|
 phosphate
phosphate
|
+
|
H(+)
|
|
 |
 |
 |
 |
 |
 |
 |
 |
 |
 |
|
Molecule diagrams generated from .mol files obtained from the
KEGG ftp site
|
|
 |
 |
 |
 |
 |
 |
 |
 |
 |
 |
 |
 |
 |
 |
 |

|

|

|
| |
|
|
| |
|
|
Sci Rep
7:5831
(2017)
|
|
PubMed id:
|
|
|
|

|
| |
|
An engineered protein antagonist of K-Ras/B-Raf interaction.
|
|
M.J.Kauke,
M.W.Traxlmayr,
J.A.Parker,
J.D.Kiefer,
R.Knihtila,
J.McGee,
G.Verdine,
C.Mattos,
K.D.Wittrup.
|
|
|

|
| |
ABSTRACT
|
|

|
| |

|
Ras is at the hub of signal transduction pathways controlling cell proliferation
and survival. Its mutants, present in about 30% of human cancers, are major
drivers of oncogenesis and render tumors unresponsive to standard therapies.
Here we report the engineering of a protein scaffold for preferential binding to
K-Ras G12D. This is the first reported inhibitor to achieve nanomolar affinity
while exhibiting specificity for mutant over wild type (WT) K-Ras. Crystal
structures of the protein R11.1.6 in complex with K-Ras WT and K-Ras G12D offer
insight into the structural basis for specificity, highlighting differences in
the switch I conformation as the major defining element in the higher affinity
interaction. R11.1.6 directly blocks interaction with Raf and reduces signaling
through the Raf/MEK/ERK pathway. Our results support greater consideration of
the state of switch I and provide a novel tool to study Ras biology. Most
importantly, this work makes an unprecedented contribution to Ras research in
inhibitor development strategy by revealing details of a targetable binding
surface. Unlike the polar interfaces found for Ras/effector interactions, the
K-Ras/R11.1.6 complex reveals an extensive hydrophobic interface that can serve
as a template to advance the development of high affinity, non-covalent
inhibitors of K-Ras oncogenic mutants.

|

|
|
|

|

|
|
 |
 |
|
 |
 |
 |
 |
 |
 |
 |
 |
 |
 |
|

