 |
PDBsum entry 5ixc

|
|
|

|
 |

|

|

|

|

|

|

|

|

|

|

|

|

|

|

|

|

|

|

|

|
 |

|

|

|
 |
 |
 |
 |
 |
 |
 |
 |
 |
 |
 |
 |
 |
 |
 |
 |
 |
 |
 |
 |
 |

|

|

|

|

|

|

|

|

|
|
Hydrolase/hydrolase inhibitor
|
PDB id
|
|

|

|
5ixc
|

|

|

|

|

|

|

|

|

|

|

|

|

|

|

|

|
 |
|
|
 |
 |
 |
 |
Enzyme class:
|
 |
E.C.3.1.1.4
- phospholipase A2.
|
|
 |
 |
 |
 |
 |
Reaction:
|
 |
a 1,2-diacyl-sn-glycero-3-phosphocholine + H2O = a 1-acyl-sn-glycero-3- phosphocholine + a fatty acid + H+
|
 |
 |
 |
 |
 |
 1,2-diacyl-sn-glycero-3-phosphocholine
1,2-diacyl-sn-glycero-3-phosphocholine
|
+
|
H2O
|
=
|
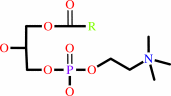 1-acyl-sn-glycero-3- phosphocholine
1-acyl-sn-glycero-3- phosphocholine
|
+
|
 fatty acid
fatty acid
|
+
|
H(+)
|
|
 |
 |
 |
 |
 |
 |
 |
 |
Cofactor:
|
 |
Ca(2+)
|
 |
 |
 |
 |
 |
 |
 |
|
Molecule diagrams generated from .mol files obtained from the
KEGG ftp site
|
|
 |
 |
 |
 |
 |
 |
 |
 |
 |
 |
 |
 |
 |
 |
 |

|

|

|
| |
|
|
| |
|
DOI no:
|
J Mol Biol
428:2769-2779
(2016)
|
|
PubMed id:
|
|
|
|

|
| |
|
Structure of Human GIVD Cytosolic Phospholipase A2 Reveals Insights into Substrate Recognition.
|
|
H.Wang,
M.G.Klein,
G.Snell,
W.Lane,
H.Zou,
I.Levin,
K.Li,
B.C.Sang.
|
|
|

|
| |
ABSTRACT
|
|

|
| |

|
Cytosolic phospholipases A2 (cPLA2s) consist of a family of calcium-sensitive
enzymes that function to generate lipid second messengers through hydrolysis of
membrane-associated glycerophospholipids. The GIVD cPLA2 (cPLA2δ) is a
potential drug target for developing a selective therapeutic agent for the
treatment of psoriasis. Here, we present two X-ray structures of human cPLA2δ,
capturing an apo state, and in complex with a substrate-like inhibitor.
Comparison of the apo and inhibitor-bound structures reveals conformational
changes in a flexible cap that allows the substrate to access the relatively
buried active site, providing new insight into the mechanism for substrate
recognition. The cPLA2δ structure reveals an unexpected second C2 domain that
was previously unrecognized from sequence alignments, placing cPLA2δ into the
class of membrane-associated proteins that contain a tandem pair of C2 domains.
Furthermore, our structures elucidate novel inter-domain interactions and define
three potential calcium-binding sites that are likely important for regulation
and activation of enzymatic activity. These findings provide novel insights into
the molecular mechanisms governing cPLA2's function in signal transduction.

|

|
|
|

|

|
|
 |
 |
|
 |
 |
 |
 |
 |
 |
 |
 |
 |
 |

