 |
PDBsum entry 4xvd

|
|
|

|
 |

|

|

|

|

|

|

|

|

|

|

|

|

|

|

|

|

|

|
 |

|

|

|
 |
 |
 |
 |
 |
 |
 |
 |
 |
 |
 |
 |
 |
 |
 |
 |
 |
 |
 |
 |

|

|

|

|

|

|

|

|

|
|
Oxidoreductase/oxidoreductase inhibitor
|
PDB id
|
|

|

|
4xvd
|

|

|

|

|

|

|

|

|

|

|

|

|

|

|

|

|
 |
|
|
 |
 |
 |
 |
Enzyme class 1:
|
 |
E.C.1.1.1.188
- prostaglandin-F synthase.
|
|
 |
 |
 |
 |
 |
Reaction:
|
 |
prostaglandin F2alpha + NADP+ = prostaglandin D2 + NADPH + H+
|
 |
 |
 |
 |
 |
prostaglandin F2alpha
Bound ligand (Het Group name = )
corresponds exactly
|
+
|
 NADP(+)
NADP(+)
|
=
|
 prostaglandin D2
prostaglandin D2
|
+
|
 NADPH
NADPH
|
+
|
H(+)
|
|
 |
 |
 |
 |
 |
 |
 |
 |
Enzyme class 2:
|
 |
E.C.1.1.1.210
- 3beta-(or 20alpha)-hydroxysteroid dehydrogenase.
|
|
 |
 |
 |
 |
 |
Reaction:
|
 |
5alpha-androstane-3beta,17beta-diol + NADP+ = 17beta-hydroxy-5alpha- androstan-3-one + NADPH + H+
|
 |
 |
 |
 |
 |
5alpha-androstane-3beta,17beta-diol
|
+
|
NADP(+)
Bound ligand (Het Group name = )
corresponds exactly
|
=
|
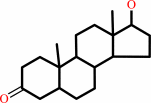 17beta-hydroxy-5alpha- androstan-3-one
17beta-hydroxy-5alpha- androstan-3-one
|
+
|
 NADPH
NADPH
|
+
|
H(+)
|
|
 |
 |
 |
 |
 |
 |
 |
 |
Enzyme class 3:
|
 |
E.C.1.1.1.239
- 3alpha-(17beta)-hydroxysteroid dehydrogenase (NAD(+)).
|
|
 |
 |
 |
 |
 |
Reaction:
|
 |
testosterone + NAD+ = androst-4-ene-3,17-dione + NADH + H+
|
 |
 |
 |
 |
 |
testosterone
Bound ligand (Het Group name = )
matches with 91.67% similarity
|
+
|
 NAD(+)
NAD(+)
|
=
|
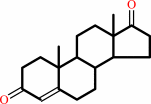 androst-4-ene-3,17-dione
androst-4-ene-3,17-dione
|
+
|
 NADH
NADH
|
+
|
H(+)
|
|
 |
 |
 |
 |
 |
 |
 |
 |
Enzyme class 4:
|
 |
E.C.1.1.1.357
- 3alpha-hydroxysteroid 3-dehydrogenase.
|
|
 |
 |
 |
 |
 |
Reaction:
|
 |
|
1.
|
a 3alpha-hydroxysteroid + NADP+ = a 3-oxosteroid + NADPH + H+
|
|
2.
|
a 3alpha-hydroxysteroid + NAD+ = a 3-oxosteroid + NADH + H+
|
|
 |
 |
 |
 |
 |
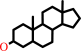 3alpha-hydroxysteroid
3alpha-hydroxysteroid
|
+
|
NADP(+)
Bound ligand (Het Group name = )
corresponds exactly
|
=
|
 3-oxosteroid
3-oxosteroid
|
+
|
 NADPH
NADPH
|
+
|
H(+)
|
|
 |
 |
 |
 |
 |
 3alpha-hydroxysteroid
3alpha-hydroxysteroid
|
+
|
NAD(+)
Bound ligand (Het Group name = )
matches with 91.67% similarity
|
=
|
 3-oxosteroid
3-oxosteroid
|
+
|
 NADH
NADH
|
+
|
H(+)
|
|
 |
 |
 |
 |
 |
 |
 |
 |
Enzyme class 5:
|
 |
E.C.1.1.1.53
- 3alpha(or 20beta)-hydroxysteroid dehydrogenase.
|
|
 |
 |
 |
 |
 |
Reaction:
|
 |
androstan-3alpha,17beta-diol + NAD+ = 17beta-hydroxyandrostanone + NADH + H+
|
 |
 |
 |
 |
 |
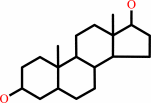 androstan-3alpha,17beta-diol
androstan-3alpha,17beta-diol
|
+
|
NAD(+)
Bound ligand (Het Group name = )
matches with 91.67% similarity
|
=
|
17beta-hydroxyandrostanone
|
+
|
 NADH
NADH
|
+
|
H(+)
|
|
 |
 |
 |
 |
 |
 |
 |
 |
Enzyme class 6:
|
 |
E.C.1.1.1.62
- 17beta-estradiol 17-dehydrogenase.
|
|
 |
 |
 |
 |
 |
Reaction:
|
 |
|
1.
|
17beta-estradiol + NAD+ = estrone + NADH + H+
|
|
2.
|
17beta-estradiol + NADP+ = estrone + NADPH + H+
|
|
 |
 |
 |
 |
 |
17beta-estradiol
|
+
|
NAD(+)
Bound ligand (Het Group name = )
matches with 91.67% similarity
|
=
|
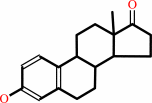 estrone
estrone
|
+
|
 NADH
NADH
|
+
|
H(+)
|
|
 |
 |
 |
 |
 |
17beta-estradiol
|
+
|
NADP(+)
Bound ligand (Het Group name = )
corresponds exactly
|
=
|
 estrone
estrone
|
+
|
 NADPH
NADPH
|
+
|
H(+)
|
|
 |
 |
 |
 |
 |
 |
 |
 |
Enzyme class 7:
|
 |
E.C.1.1.1.64
- testosterone 17beta-dehydrogenase (NADP(+)).
|
|
 |
 |
 |
 |
 |
Reaction:
|
 |
testosterone + NADP+ = androst-4-ene-3,17-dione + NADPH + H+
|
 |
 |
 |
 |
 |
testosterone
Bound ligand (Het Group name = )
corresponds exactly
|
+
|
 NADP(+)
NADP(+)
|
=
|
 androst-4-ene-3,17-dione
androst-4-ene-3,17-dione
|
+
|
 NADPH
NADPH
|
+
|
H(+)
|
|
 |
 |
 |
 |
 |
 |
 |
 |
 |
 |
|
Note, where more than one E.C. class is given (as above), each may
correspond to a different protein domain or, in the case of polyprotein
precursors, to a different mature protein.
|
|
 |
|
Molecule diagrams generated from .mol files obtained from the
KEGG ftp site
|
|
 |
 |
 |
 |
 |
 |
 |
 |
 |
 |
 |
 |
 |
 |
 |

|

|

|
| |
|
|
| |
|
DOI no:
|
Acta Crystallogr D Biol Crystallogr
71:918-927
(2015)
|
|
PubMed id:
|
|
|
|

|
| |
|
Structures of complexes of type 5 17β-hydroxysteroid dehydrogenase with structurally diverse inhibitors: insights into the conformational changes upon inhibitor binding.
|
|
Y.Amano,
T.Yamaguchi,
T.Niimi,
H.Sakashita.
|
|
|

|
| |
ABSTRACT
|
|

|
| |

|
Type 5 17β-hydroxysteroid dehydrogenase (17β-HSD5) is an aldo-keto reductase
expressed in the human prostate which catalyzes the conversion of
androstenedione to testosterone. Testosterone is converted to
5α-dihydrotestosterone, which is present at high concentrations in patients
with castration-resistant prostate cancer (CRPC). Inhibition of 17β-HSD5 is
therefore considered to be a promising therapy for treating CRPC. In the present
study, crystal structures of complexes of 17β-HSD5 with structurally diverse
inhibitors derived from high-throughput screening were determined. In the
structures of the complexes, various functional groups, including amide, nitro,
pyrazole and hydroxyl groups, form hydrogen bonds to the catalytic residues
His117 and Tyr55. In addition, major conformational changes of 17β-HSD5 were
observed following the binding of the structurally diverse inhibitors. These
results demonstrate interactions between 17β-HSD5 and inhibitors at the atomic
level and enable structure-based drug design for anti-CRPC therapy.

|

|
|
|

|

|
|
 |
 |
|
 |
 |
 |
 |
 |
 |
 |
 |
 |
 |

