 |
PDBsum entry 4bf6
|
|

|
|
PDB id:
|
 |
|
 |
| Name: |
 |
Lyase
|
 |
|
Title:
|
 |
Three dimensional structure of human carbonic anhydrase ii in complex with 5-(1-(3-cyanophenyl)-1h-1,2,3-triazol-4-yl)thiophene-2- sulfonamide
|
|
Structure:
|
 |
Carbonic anhydrase 2. Chain: a. Synonym: carbonate dehydratase ii, carbonic anhydrasE C, cac, carbonic anhydrase ii, ca-ii. Engineered: yes
|
|
Source:
|
 |
Homo sapiens. Organism_taxid: 9606. Expressed in: escherichia coli. Expression_system_taxid: 469008.
|
|
Resolution:
|
 |
|
1.82Å
|
R-factor:
|
0.148
|
R-free:
|
0.202
|
|
|
Authors:
|
 |
K.Tars,J.Leitans,R.Zalubovskis
|
|
Key ref:
|
 |
J.Leitans
et al.
(2013).
5-Substituted-(1,2,3-triazol-4-yl)thiophene-2-sulfonamides strongly inhibit human carbonic anhydrases I, II, IX and XII: solution and X-ray crystallographic studies.
Bioorg Med Chem Lett,
21,
5130-5138.
PubMed id:
DOI:
|
 |
|
Date:
|
 |
|
15-Mar-13
|
Release date:
|
22-Jan-14
|
|
|

|

|
|
PROCHECK
|

|

|
|
|
Headers
|
 |
|
|
References
|
|
|
|

|

|
|
P00918
(CAH2_HUMAN) -
Carbonic anhydrase 2 from Homo sapiens
|
|

|

|
Seq:
Struc:
|
 |
 |
 |
260 a.a.
257 a.a.
|

|

|

|

|
|

|

|

|
 |
 |

|
|
Key: |
 |
PfamA domain |
 |
 |
 |
Secondary structure |
 |
 |
CATH domain |
 |
|

|
|
|
 |
|
|
 |
 |
 |
 |
Enzyme class 2:
|
 |
E.C.4.2.1.1
- carbonic anhydrase.
|
|
 |
 |
 |
 |
 |
Reaction:
|
 |
hydrogencarbonate + H+ = CO2 + H2O
|
 |
 |
 |
 |
 |
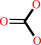 hydrogencarbonate
hydrogencarbonate
|
+
|
H(+)
|
=
|
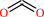 CO2
CO2
|
+
|
H2O
|
|
 |
 |
 |
 |
 |
 |
 |
 |
Cofactor:
|
 |
Zn(2+)
|
 |
 |
 |
 |
 |
Enzyme class 3:
|
 |
E.C.4.2.1.69
- cyanamide hydratase.
|
|
 |
 |
 |
 |
 |
Reaction:
|
 |
urea = cyanamide + H2O
|
 |
 |
 |
 |
 |
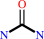 urea
urea
|
=
|
 cyanamide
cyanamide
|
+
|
H2O
|
|
 |
 |
 |
 |
 |
 |
 |
 |
 |
 |
|
Note, where more than one E.C. class is given (as above), each may
correspond to a different protein domain or, in the case of polyprotein
precursors, to a different mature protein.
|
|
 |
|
Molecule diagrams generated from .mol files obtained from the
KEGG ftp site
|
|
 |
 |
 |
 |
 |
 |
 |
 |
 |
 |
 |
 |
 |
 |
 |

|

|

|
| |
|
|
| |
|
DOI no:
|
Bioorg Med Chem Lett
21:5130-5138
(2013)
|
|
PubMed id:
|
|
|
|

|
| |
|
5-Substituted-(1,2,3-triazol-4-yl)thiophene-2-sulfonamides strongly inhibit human carbonic anhydrases I, II, IX and XII: solution and X-ray crystallographic studies.
|
|
J.Leitans,
A.Sprudza,
M.Tanc,
I.Vozny,
R.Zalubovskis,
K.Tars,
C.T.Supuran.
|
|
|

|
| |
ABSTRACT
|
|

|
| |

|
We report here a series of 2-thiophene-sulfonamides incorporating 1-substituted
aryl-1,2,3-triazolyl moieties, prepared by click chemistry from
5-ethynylthiophene-2-sulfonamide and substituted aryl azides. The new
sulfonamides were investigated as inhibitors of the zinc metalloenzyme CA (EC
4.2.1.1), and more specifically against the human (h) cytosolic isoforms hCA I
and II and the transmembrane, tumor-associated ones hCA IX and XII: The new
compounds were medium-weak hCA I inhibitors (KIs in the range of 224-7544nM),
but were compactly, highly effective, low nanomolar hCA II inhibitors (KIs of
2.2-7.7nM). The tumor-associated hCA IX was inhibited with KIs ranging between
5.4 and 811nM, whereas hCA XII with inhibition constants in the range of
3.4-239nM. The X-ray crystal structure of the adducts of two such compounds
bound to hCA II (one incorporating 1-naphthyl, the other one 3-cyanophenyl
moieties) evidenced the reasons of the high affinity for hCA II. Highly
favorable, predominantly hydrophobic interactions between the sulfonamide
scaffold and the hCA II active site were responsible for the binding, in
addition to the coordination of the sulfamoyl moiety to the zinc ion. The tails
of the two inhibitors adopted very diverse orientations when bound to the active
site, with the naphthyltriazolyl moiety orientated towards the hydrophobic half
of the active site, and the 3-cyanophenyl one pointing towards the hydrophilic
half. These data may be used for the structure-based drug design of even more
effective hCA II inhibitors, with potential use as antiglaucoma agents or as
diuretics.

|

|
|
|

|

|
|
 |
 |
|
 |
 |
 |
 |
 |
 |
 |
 |
 |
 |

