 |
PDBsum entry 3e3h

|

|

|
|
 |
Contents |
 |
|
|

|

|
|
|
|
|
|
|
|
|
|
* Residue conservation analysis
|
|
|
|
 |
|
|
 |
 |
 |
 |
Enzyme class:
|
 |
E.C.3.1.8.1
- aryldialkylphosphatase.
|
|
 |
 |
 |
 |
 |
Reaction:
|
 |
An aryl dialkyl phosphate + H2O = dialkyl phosphate + an aryl alcohol
|
 |
 |
 |
 |
 |
 aryl dialkyl phosphate
aryl dialkyl phosphate
|
+
|
H2O
|
=
|
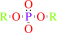 dialkyl phosphate
dialkyl phosphate
|
+
|
aryl alcohol
|
|
 |
 |
 |
 |
 |
 |
 |
 |
Cofactor:
|
 |
Divalent cation
|
 |
 |
 |
 |
 |
 |
 |
|
Molecule diagrams generated from .mol files obtained from the
KEGG ftp site
|
|
 |
 |
 |
 |
 |
 |
 |
 |
 |
 |
 |
 |
 |
 |
 |

|

|

|
| |
|
|
| |
|
|
Protein Eng Des Sel
21:405-412
(2008)
|
|
PubMed id:
|
|
|
|

|
| |
|
Balancing the stability and the catalytic specificities of OP hydrolases with enhanced V-agent activities.
|
|
T.E.Reeves,
M.E.Wales,
J.K.Grimsley,
P.Li,
D.M.Cerasoli,
J.R.Wild.
|
|
|

|
| |
ABSTRACT
|
|

|
| |

|
Rational site-directed mutagenesis and biophysical analyses have been used to
explore the thermodynamic stability and catalytic capabilities of
organophosphorus hydrolase (OPH) and its genetically modified variants. There
are clear trade-offs in the stability of modifications that enhance catalytic
activities. For example, the H254R/H257L variant has higher turnover numbers for
the chemical warfare agents VX (144 versus 14 s(-1) for the native enzyme (wild
type) and VR (Russian VX, 465 versus 12 s(-1) for wild type). These increases
are accompanied by a loss in stability in which the total Gibb's free energy for
unfolding is 19.6 kcal/mol, which is 5.7 kcal/mol less than that of the
wild-type enzyme. X-ray crystallographic studies support biophysical data that
suggest amino acid residues near the active site contribute to the chemical and
thermal stability through hydrophobic and cation-pi interactions. The cation-pi
interactions appear to contribute an additional 7 kcal/mol to the overall global
stability of the enzyme. Using rational design, it has been possible to make
amino acid changes in this region that restored the stability, yet maintained
effective V-agent activities, with turnover numbers of 68 and 36 s(-1) for VX
and VR, respectively. This study describes the first rationally designed,
stability/activity balance for an OPH enzyme with a legitimate V-agent activity,
and its crystal structure.

|

|
|
|

|

|
|
 |
 |
|
 |
 |
 |
 |
 |
 |
 |
 |
 |
 |

