 |
PDBsum entry 3cs2

|
|
|

|
 |

|

|

|

|

|

|

|

|

|

|

|

|

|

|

|

|

|

|
 |

|

|

|
 |
 |
 |
 |
 |
 |
 |
 |
 |
 |
 |
 |
 |
 |
 |
 |
 |
 |
 |
 |

|

|

|

|

|

|

|

|

|
|
Hydrolase, metal binding protein
|
PDB id
|
|

|

|
3cs2
|

|

|

|

|

|

|

|

|

|

|

|

|

|

|

|

|

|

|

|
|
 |
Contents |
 |
|
|

|

|
|
|
|
|
|
|
|
|
|
* Residue conservation analysis
|
|
|
|
 |
|
|
 |
 |
 |
 |
Enzyme class:
|
 |
E.C.3.1.8.1
- aryldialkylphosphatase.
|
|
 |
 |
 |
 |
 |
Reaction:
|
 |
An aryl dialkyl phosphate + H2O = dialkyl phosphate + an aryl alcohol
|
 |
 |
 |
 |
 |
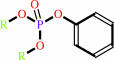 aryl dialkyl phosphate
aryl dialkyl phosphate
|
+
|
H2O
|
=
|
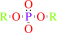 dialkyl phosphate
dialkyl phosphate
|
+
|
aryl alcohol
|
|
 |
 |
 |
 |
 |
 |
 |
 |
Cofactor:
|
 |
Divalent cation
|
 |
 |
 |
 |
 |
 |
 |
|
Molecule diagrams generated from .mol files obtained from the
KEGG ftp site
|
|
 |
 |
 |
 |
 |
 |
 |
 |
 |
 |
 |
 |
 |
 |
 |

|

|

|
| |
|
|
| |
|
|
Biochemistry
47:9497-9504
(2008)
|
|
PubMed id:
|
|
|
|

|
| |
|
Structure of diethyl phosphate bound to the binuclear metal center of phosphotriesterase.
|
|
J.Kim,
P.C.Tsai,
S.L.Chen,
F.Himo,
S.C.Almo,
F.M.Raushel.
|
|
|

|
| |
ABSTRACT
|
|

|
| |

|
The bacterial phosphotriesterase (PTE) from Pseudomonas diminuta catalyzes the
hydrolysis of organophosphate esters at rates close to the diffusion limit.
X-ray diffraction studies have shown that a binuclear metal center is positioned
in the active site of PTE and that this complex is responsible for the
activation of the nucleophilic water from solvent. In this paper, the
three-dimensional structure of PTE was determined in the presence of the
hydrolysis product, diethyl phosphate (DEP), and a product analogue, cacodylate.
In the structure of the PTE-diethyl phosphate complex, the DEP product is found
symmetrically bridging the two divalent cations. The DEP displaces the hydroxide
from solvent that normally bridges the two divalent cations in structures
determined in the presence or absence of substrate analogues. One of the
phosphoryl oxygen atoms in the PTE-DEP complex is 2.0 A from the alpha-metal
ion, while the other oxygen is 2.2 A from the beta-metal ion. The two metal ions
are separated by a distance of 4.0 A. A similar structure is observed in the
presence of cacodylate. Analogous complexes have previously been observed for
the product complexes of isoaspartyl dipeptidase, d-aminoacylase, and
dihydroorotase from the amidohydrolase superfamily of enzymes. The
experimentally determined structure of the PTE-diethyl phosphate product complex
is inconsistent with a recent proposal based upon quantum mechanical/molecular
mechanical simulations which postulated the formation of an asymmetrical product
complex bound exclusively to the beta-metal ion with a metal-metal separation of
5.3 A. This structure is also inconsistent with a chemical mechanism for
substrate hydrolysis that utilizes the bridging hydroxide as a base to abstract
a proton from a water molecule loosely associated with the alpha-metal ion.
Density functional theory (DFT) calculations support a reaction mechanism that
utilizes the bridging hydroxide as the direct nucleophile in the hydrolysis of
organophosphate esters by PTE.

|

|
|
|

|

|
|
 |
 |
|
 |
 |
 |
 |
 |
 |
 |
 |
 |
|
Literature references that cite this PDB file's key reference
|
|
 |
| |
PubMed id
|
 |
Reference
|
 |
|
|
|
 |
L.Briseño-Roa,
C.M.Timperley,
A.D.Griffiths,
and
A.R.Fersht
(2011).
Phosphotriesterase variants with high methylphosphonatase activity and strong negative trade-off against phosphotriesters.
|
| |
Protein Eng Des Sel,
24,
151-159.
|
 |
|
|
|
|
 |
F.Ely,
K.S.Hadler,
L.R.Gahan,
L.W.Guddat,
D.L.Ollis,
and
G.Schenk
(2010).
The organophosphate-degrading enzyme from Agrobacterium radiobacter displays mechanistic flexibility for catalysis.
|
| |
Biochem J,
432,
565-573.
|
 |
|
PDB codes:
|
 |
|
|
|
|
|
 |
D.F.Xiang,
C.Xu,
D.Kumaran,
A.C.Brown,
J.M.Sauder,
S.K.Burley,
S.Swaminathan,
and
F.M.Raushel
(2009).
Functional annotation of two new carboxypeptidases from the amidohydrolase superfamily of enzymes.
|
| |
Biochemistry,
48,
4567-4576.
|
 |
|
|
|
|
 |
S.C.Kamerlin,
M.Haranczyk,
and
A.Warshel
(2009).
Progress in ab initio QM/MM free-energy simulations of electrostatic energies in proteins: accelerated QM/MM studies of pKa, redox reactions and solvation free energies.
|
| |
J Phys Chem B,
113,
1253-1272.
|
 |
|
|
|
|
 |
X.Zhang,
R.Wu,
L.Song,
Y.Lin,
M.Lin,
Z.Cao,
W.Wu,
and
Y.Mo
(2009).
Molecular dynamics simulations of the detoxification of paraoxon catalyzed by phosphotriesterase.
|
| |
J Comput Chem,
30,
2388-2401.
|
 |
|
 |
 |
|
The most recent references are shown first.
Citation data come partly from CiteXplore and partly
from an automated harvesting procedure. Note that this is likely to be
only a partial list as not all journals are covered by
either method. However, we are continually building up the citation data
so more and more references will be included with time.
Where a reference describes a PDB structure, the PDB
codes are
shown on the right.
|
 |

