 |
PDBsum entry 2wwh

|

|

|
|
 |
Contents |
 |
|
|

|

|
|
|
|
|
|
|
|
|
|
* Residue conservation analysis
|
|
|

|
|
PDB id:
|
 |
|
 |
| Name: |
 |
Transferase
|
 |
|
Title:
|
 |
Plasmodium falciparum thymidylate kinase in complex with ap5dt
|
|
Structure:
|
 |
Thymidilate kinase, putative. Chain: a, b, c. Engineered: yes
|
|
Source:
|
 |
Plasmodium falciparum. Organism_taxid: 36329. Strain: 3d7. Expressed in: escherichia coli. Expression_system_taxid: 469008.
|
|
Resolution:
|
 |
|
2.70Å
|
R-factor:
|
0.191
|
R-free:
|
0.270
|
|
|
Authors:
|
 |
J.L.Whittingham,J.Carrero-Lerida,J.A.Brannigan,L.M.Ruiz-Perez, A.P.G.Silva,M.J.Fogg,A.J.Wilkinson,I.H.Gilbert,K.S.Wilson, D.Gonzalez-Pacanowska
|
|
Key ref:
|
 |
J.L.Whittingham
et al.
(2010).
Structural basis for the efficient phosphorylation of AZT-MP (3'-azido-3'-deoxythymidine monophosphate) and dGMP by Plasmodium falciparum type I thymidylate kinase.
Biochem J,
428,
499-509.
PubMed id:
|
 |
|
Date:
|
 |
|
23-Oct-09
|
Release date:
|
21-Apr-10
|
|
|

|

|
|
PROCHECK
|

|

|
|
|
Headers
|
 |
|
|
References
|
|
|
|

|

|
|
Q8I4S1
(Q8I4S1_PLAF7) -
Thymidylate kinase from Plasmodium falciparum (isolate 3D7)
|
|

|

|
Seq:
Struc:
|
 |
 |
 |
210 a.a.
211 a.a.
|

|

|

|

|
|

|

|

|
 |
 |

|
|
Key: |
 |
PfamA domain |
 |
 |
 |
Secondary structure |
 |
 |
CATH domain |
 |
|

|
|
|
 |
|
|
 |
 |
 |
 |
Enzyme class 2:
|
 |
E.C.2.7.4.8
- guanylate kinase.
|
|
 |
 |
 |
 |
 |
Reaction:
|
 |
GMP + ATP = GDP + ADP
|
 |
 |
 |
 |
 |
GMP
Bound ligand (Het Group name = )
matches with 56.36% similarity
|
+
|
 ATP
ATP
|
=
|
 GDP
GDP
|
+
|
 ADP
ADP
|
|
 |
 |
 |
 |
 |
 |
 |
 |
Enzyme class 3:
|
 |
E.C.2.7.4.9
- dTMP kinase.
|
|
 |
 |
 |
 |
 |
Reaction:
|
 |
dTMP + ATP = dTDP + ADP
|
 |
 |
 |
 |
 |
dTMP
Bound ligand (Het Group name = )
matches with 56.36% similarity
|
+
|
 ATP
ATP
|
=
|
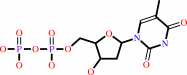 dTDP
dTDP
|
+
|
 ADP
ADP
|
|
 |
 |
 |
 |
 |
 |
 |
 |
 |
 |
|
Note, where more than one E.C. class is given (as above), each may
correspond to a different protein domain or, in the case of polyprotein
precursors, to a different mature protein.
|
|
 |
|
Molecule diagrams generated from .mol files obtained from the
KEGG ftp site
|
|
 |
 |
 |
 |
 |
 |
 |
 |
 |
 |
 |
 |
 |
 |
 |

|

|

|
| |
|
|
| |
|
|
Biochem J
428:499-509
(2010)
|
|
PubMed id:
|
|
|
|

|
| |
|
Structural basis for the efficient phosphorylation of AZT-MP (3'-azido-3'-deoxythymidine monophosphate) and dGMP by Plasmodium falciparum type I thymidylate kinase.
|
|
J.L.Whittingham,
J.Carrero-Lerida,
J.A.Brannigan,
L.M.Ruiz-Perez,
A.P.Silva,
M.J.Fogg,
A.J.Wilkinson,
I.H.Gilbert,
K.S.Wilson,
D.González-Pacanowska.
|
|
|

|
| |
ABSTRACT
|
|

|
| |

|
Plasmodium falciparum is the causative agent of malaria, a disease where new
drug targets are required due to increasing resistance to current
anti-malarials. TMPK (thymidylate kinase) is a good candidate as it is essential
for the synthesis of dTTP, a critical precursor of DNA and has been much studied
due to its role in prodrug activation and as a drug target. Type I TMPKs, such
as the human enzyme, phosphorylate the substrate AZT
(3'-azido-3'-deoxythymidine)-MP (monophosphate) inefficiently compared with type
II TMPKs (e.g. Escherichia coli TMPK). In the present paper we report that
eukaryotic PfTMPK (P. falciparum TMPK) presents sequence features of a type I
enzyme yet the kinetic parameters for AZT-MP phosphorylation are similar to
those of the highly efficient E. coli enzyme. Structural information shows that
this is explained by a different juxtaposition of the P-loop and the azide of
AZT-MP. Subsequent formation of the transition state requires no further
movement of the PfTMPK P-loop, with no steric conflicts for the azide moiety,
allowing efficient phosphate transfer. Likewise, we present results that confirm
the ability of the enzyme to uniquely accept dGMP as a substrate and shed light
on the basis for its wider substrate specificity. Information resulting from two
ternary complexes (dTMP-ADP and AZT-MP-ADP) and a binary complex with the
transition state analogue AP5dT [P1-(5'-adenosyl)-P5-(5'-thymidyl)
pentaphosphate] all reveal significant differences with the human enzyme,
notably in the lid region and in the P-loop which may be exploited in the
rational design of Plasmodium-specific TMPK inhibitors with therapeutic
potential.

|

|
|
|

|

|
|
 |
 |
|
 |
 |
 |
 |
 |
 |
 |
 |
 |
 |

