 |
PDBsum entry 2jfc

|
|
|

|
 |

|

|

|

|

|

|

|

|

|

|

|

|

|

|

|

|
 |

|

|

|
 |
 |
 |
 |
 |
 |
 |
 |
 |
 |
 |
 |
 |
 |
 |
 |
 |
 |
 |

|

|

|

|

|

|

|

|

|
|
Oxidoreductase
|
PDB id
|
|

|

|
2jfc
|

|

|

|

|

|

|

|

|

|

|

|

|

|

|

|

|

|

|

|
|
 |
Contents |
 |
|
|

|

|
|
|
|
|
|
|
|
* Residue conservation analysis
|
|
|

|
|
PDB id:
|
 |
|
 |
| Name: |
 |
Oxidoreductase
|
 |
|
Title:
|
 |
M144l mutant of nitrite reductase from alcaligenes xylosoxidans in space group p212121
|
|
Structure:
|
 |
Dissimilatory copper-containing nitrite reductase. Chain: a, b, c, d, e, f. Fragment: residues 26-360. Synonym: nitrite reductase, nir. Engineered: yes. Mutation: yes
|
|
Source:
|
 |
Achromobacter xylosoxidans. Organism_taxid: 85698. Expressed in: escherichia coli. Expression_system_taxid: 562
|
|
Resolution:
|
 |
|
2.40Å
|
R-factor:
|
0.172
|
R-free:
|
0.193
|
|
|
Authors:
|
 |
K.Paraskevopoulos,M.A.Hough,R.G.Sawers,R.R.Eady,S.S.Hasnain
|
|
Key ref:
|
 |
K.Paraskevopoulos
et al.
(2007).
The structure of the Met144Leu mutant of copper nitrite reductase from Alcaligenes xylosoxidans provides the first glimpse of a protein-protein complex with azurin II.
J Biol Inorg Chem,
12,
789-796.
PubMed id:
|
 |
|
Date:
|
 |
|
31-Jan-07
|
Release date:
|
29-May-07
|
|
|

|

|
|
PROCHECK
|

|

|
|
|
Headers
|
 |
|
|
References
|
|
|
|

|

|
|
O68601
(O68601_ALCXX) -
Copper-containing nitrite reductase from Alcaligenes xylosoxydans xylosoxydans
|
|

|

|
Seq:
Struc:
|
 |
 |
 |
360 a.a.
335 a.a.*
|

|

|

|

|
|

|

|

|
 |
 |

|
|
Key: |
 |
 |
Secondary structure |
 |
 |
CATH domain |
 |
|
*
PDB and UniProt seqs differ
at 1 residue position (black
cross)
|
|

|
|
|
 |
|
|
 |
 |
 |
 |
Enzyme class:
|
 |
E.C.1.7.2.1
- nitrite reductase (NO-forming).
|
|
 |
 |
 |
 |
 |
Reaction:
|
 |
nitric oxide + Fe(III)-[cytochrome c] + H2O = Fe(II)-[cytochrome c] + nitrite + 2 H+
|
 |
 |
 |
 |
 |
 nitric oxide
nitric oxide
|
+
|
Fe(III)-[cytochrome c]
|
+
|
H2O
|
=
|
Fe(II)-[cytochrome c]
|
+
|
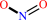 nitrite
nitrite
|
+
|
2
×
H(+)
|
|
 |
 |
 |
 |
 |
 |
 |
 |
Cofactor:
|
 |
Cu cation or Fe cation; FAD
|
 |
 |
 |
 |
 |
Cu cation
|
or
|
Fe cation
|
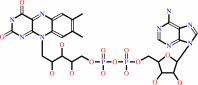 FAD
FAD
|
|
 |
 |
 |
 |
|
Molecule diagrams generated from .mol files obtained from the
KEGG ftp site
|
|
 |
 |
 |
 |
 |
 |
 |
 |
 |
 |
 |
 |
 |
 |
 |

|

|

|
| |
|
|
| |
|
|
J Biol Inorg Chem
12:789-796
(2007)
|
|
PubMed id:
|
|
|
|

|
| |
|
The structure of the Met144Leu mutant of copper nitrite reductase from Alcaligenes xylosoxidans provides the first glimpse of a protein-protein complex with azurin II.
|
|
K.Paraskevopoulos,
M.A.Hough,
R.G.Sawers,
R.R.Eady,
S.S.Hasnain.
|
|
|

|
| |
ABSTRACT
|
|

|
| |

|
Cu-containing nitrite reductases (NiRs) perform the reduction of nitrite to NO
via an ordered mechanism in which the delivery of a proton and an electron to
the catalytic type 2 Cu site is highly orchestrated. Electron transfer from a
redox partner protein, azurin or pseudoazurin, to the type 1 Cu site is assumed
to occur through the formation of a protein-protein complex. We report here a
new crystal form in space group P2(1)2(1)2(1) of the Met144Leu mutant of NiR
from Alcaligenes xylosoxidans (AxNiR), revealing a head-to-head packing motif
involving residues around the hydrophobic patch of domain 1. Superposition of
the structure of azurin II with that of domain 1 of one of the Met144Leu
molecules provides the first glimpse of an azurin II-NiR protein-protein
complex. Mutations of two of the residues of AxNiR, Trp138His (Barrett et al. in
Biochemistry 43:16311-16319, 2004) and Met87Leu, highlighted in the AxNiR-azurin
complex, results in substantially decreased activity when azurin is used as the
electron donor instead of methyl viologen, providing direct evidence for the
importance of this region for complex formation.

|

|
|
|

|

|
|
 |
 |
|
 |
 |
 |
 |
 |
 |
 |
 |
 |
 |

