 |
PDBsum entry 2dln

|
|
|

|
 |

|

|

|

|

|

|

|

|

|

|

|

|

|

|
 |

|

|

|
 |
 |
 |
 |
 |
 |
 |
 |
 |
 |
 |
 |
 |
 |
 |
 |
 |
 |

|

|

|

|

|

|

|

|

|
|
Ligase(peptidoglycan synthesis)
|
PDB id
|
|

|

|
2dln
|

|

|

|

|

|

|

|

|

|

|

|

|

|

|

|

|

|

|

|
|
 |
Contents |
 |
|
|

|

|
|
|
|
|
|
|
|
|
|
* Residue conservation analysis
|
|
|
|
 |
|
|
 |
 |
 |
 |
Enzyme class:
|
 |
E.C.6.3.2.4
- D-alanine--D-alanine ligase.
|
|
 |
 |
 |
 |
 |

Pathway:
|
 |
Peptidoglycan Biosynthesis (Part 1)
|
 |
 |
 |
 |
 |
Reaction:
|
 |
2 D-alanine + ATP = D-alanyl-D-alanine + ADP + phosphate + H+
|
 |
 |
 |
 |
 |
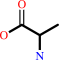 2
×
D-alanine
2
×
D-alanine
|
+
|
 ATP
ATP
|
=
|
D-alanyl-D-alanine
Bound ligand (Het Group name = )
corresponds exactly
|
+
|
 ADP
ADP
|
+
|
 phosphate
phosphate
|
+
|
H(+)
|
|
 |
 |
 |
 |
 |
 |
 |
 |
 |
 |
|
Molecule diagrams generated from .mol files obtained from the
KEGG ftp site
|
|
 |
 |
 |
 |
 |
 |
 |
 |
 |
 |
 |
 |
 |
 |
 |

|

|

|
| |
|
|
| |
|
DOI no:
|
Science
266:439-443
(1994)
|
|
PubMed id:
|
|
|
|

|
| |
|
Vancomycin resistance: structure of D-alanine:D-alanine ligase at 2.3 A resolution.
|
|
C.Fan,
P.C.Moews,
C.T.Walsh,
J.R.Knox.
|
|
|

|
| |
ABSTRACT
|
|

|
| |

|
The molecular structure of the D-alanine:D-alanine ligase of the ddlB gene of
Escherichia coli, co-crystallized with an S,R-methylphosphinate and adenosine
triphosphate, was determined by x-ray diffraction to a resolution of 2.3
angstroms. A catalytic mechanism for the ligation of two D-alanine substrates is
proposed in which a helix dipole and a hydrogen-bonded triad of tyrosine,
serine, and glutamic acid assist binding and deprotonation steps. From sequence
comparison, it is proposed that a different triad exists in a recently
discovered D-alanine:D-lactate ligase (VanA) present in vancomycin-resistant
enterococci. A molecular mechanism for the altered specificity of VanA is
suggested.

|

|
|
|

|

|
|
 |
 |
|
 |
 |
 |
 |
 |
 |
 |
 |
 |
|
Literature references that cite this PDB file's key reference
|
|
 |
| |
PubMed id
|
 |
Reference
|
 |
|
|
|
 |
A.Szyk,
A.M.Deaconescu,
G.Piszczek,
and
A.Roll-Mecak
(2011).
Tubulin tyrosine ligase structure reveals adaptation of an ancient fold to bind and modify tubulin.
|
| |
Nat Struct Mol Biol,
18,
1250-1258.
|
 |
|
PDB codes:
|
 |
|
|
|
|
|
 |
S.A.Borisova,
B.T.Circello,
J.K.Zhang,
W.A.van der Donk,
and
W.W.Metcalf
(2010).
Biosynthesis of rhizocticins, antifungal phosphonate oligopeptides produced by Bacillus subtilis ATCC6633.
|
| |
Chem Biol,
17,
28-37.
|
 |
|
|
|
|
 |
S.F.Lei,
and
J.Huan
(2010).
Towards site-based protein functional annotations.
|
| |
Int J Data Min Bioinform,
4,
452-470.
|
 |
|
|
|
|
 |
F.Depardieu,
M.L.Foucault,
J.Bell,
A.Dubouix,
M.Guibert,
J.P.Lavigne,
M.Levast,
and
P.Courvalin
(2009).
New combinations of mutations in VanD-Type vancomycin-resistant Enterococcus faecium, Enterococcus faecalis, and Enterococcus avium strains.
|
| |
Antimicrob Agents Chemother,
53,
1952-1963.
|
 |
|
|
|
|
 |
H.Li,
W.Fast,
and
S.J.Benkovic
(2009).
Structural and functional modularity of proteins in the de novo purine biosynthetic pathway.
|
| |
Protein Sci,
18,
881-892.
|
 |
|
|
|
|
 |
J.J.McGuire,
D.M.Bartley,
J.W.Tomsho,
W.H.Haile,
and
J.K.Coward
(2009).
Inhibition of human folylpolyglutamate synthetase by diastereomeric phosphinic acid mimics of the tetrahedral intermediate.
|
| |
Arch Biochem Biophys,
488,
140-145.
|
 |
|
|
|
|
 |
J.Zhan,
K.Qiao,
and
Y.Tang
(2009).
Investigation of tailoring modifications in pradimicin biosynthesis.
|
| |
Chembiochem,
10,
1447-1452.
|
 |
|
|
|
|
 |
Y.Kitamura,
A.Ebihara,
Y.Agari,
A.Shinkai,
K.Hirotsu,
and
S.Kuramitsu
(2009).
Structure of D-alanine-D-alanine ligase from Thermus thermophilus HB8: cumulative conformational change and enzyme-ligand interactions.
|
| |
Acta Crystallogr D Biol Crystallogr,
65,
1098-1106.
|
 |
|
|
|
|
 |
D.Wu,
L.Zhang,
Y.Kong,
J.Du,
S.Chen,
J.Chen,
J.Ding,
H.Jiang,
and
X.Shen
(2008).
Enzymatic characterization and crystal structure analysis of the D-alanine-D-alanine ligase from Helicobacter pylori.
|
| |
Proteins,
72,
1148-1160.
|
 |
|
PDB code:
|
 |
|
|
|
|
|
 |
H.Barreteau,
A.Kovac,
A.Boniface,
M.Sova,
S.Gobec,
and
D.Blanot
(2008).
Cytoplasmic steps of peptidoglycan biosynthesis.
|
| |
FEMS Microbiol Rev,
32,
168-207.
|
 |
|
|
|
|
 |
I.Mochalkin,
J.R.Miller,
A.Evdokimov,
S.Lightle,
C.Yan,
C.K.Stover,
and
G.L.Waldrop
(2008).
Structural evidence for substrate-induced synergism and half-sites reactivity in biotin carboxylase.
|
| |
Protein Sci,
17,
1706-1718.
|
 |
|
PDB codes:
|
 |
|
|
|
|
|
 |
S.O.Nilsson Lill,
J.Gao,
and
G.L.Waldrop
(2008).
Molecular dynamics simulations of biotin carboxylase.
|
| |
J Phys Chem B,
112,
3149-3156.
|
 |
|
|
|
|
 |
T.Arai,
and
K.Kino
(2008).
A cyanophycin synthetase from Thermosynechococcus elongatus BP-1 catalyzes primer-independent cyanophycin synthesis.
|
| |
Appl Microbiol Biotechnol,
81,
69-78.
|
 |
|
|
|
|
 |
Y.Zhang,
M.Morar,
and
S.E.Ealick
(2008).
Structural biology of the purine biosynthetic pathway.
|
| |
Cell Mol Life Sci,
65,
3699-3724.
|
 |
|
|
|
|
 |
C.H.Pai,
B.Y.Chiang,
T.P.Ko,
C.C.Chou,
C.M.Chong,
F.J.Yen,
S.Chen,
J.K.Coward,
A.H.Wang,
and
C.H.Lin
(2006).
Dual binding sites for translocation catalysis by Escherichia coli glutathionylspermidine synthetase.
|
| |
EMBO J,
25,
5970-5982.
|
 |
|
PDB codes:
|
 |
|
|
|
|
|
 |
J.H.Lee,
Y.Na,
H.E.Song,
D.Kim,
B.H.Park,
S.H.Rho,
Y.J.Im,
M.K.Kim,
G.B.Kang,
D.S.Lee,
and
S.H.Eom
(2006).
Crystal structure of the apo form of D-alanine: D-alanine ligase (Ddl) from Thermus caldophilus: a basis for the substrate-induced conformational changes.
|
| |
Proteins,
64,
1078-1082.
|
 |
|
PDB code:
|
 |
|
|
|
|
|
 |
M.Sato,
K.Kirimura,
and
K.Kino
(2006).
Substrate specificity of thermostable D-alanine-D-alanine ligase from Thermotoga maritima ATCC 43589.
|
| |
Biosci Biotechnol Biochem,
70,
2790-2792.
|
 |
|
|
|
|
 |
S.Liu,
J.S.Chang,
J.T.Herberg,
M.M.Horng,
P.K.Tomich,
A.H.Lin,
and
K.R.Marotti
(2006).
Allosteric inhibition of Staphylococcus aureus D-alanine:D-alanine ligase revealed by crystallographic studies.
|
| |
Proc Natl Acad Sci U S A,
103,
15178-15183.
|
 |
|
PDB codes:
|
 |
|
|
|
|
|
 |
A.J.McCoy,
and
A.T.Maurelli
(2005).
Characterization of Chlamydia MurC-Ddl, a fusion protein exhibiting D-alanyl-D-alanine ligase activity involved in peptidoglycan synthesis and D-cycloserine sensitivity.
|
| |
Mol Microbiol,
57,
41-52.
|
 |
|
|
|
|
 |
G.E.Besong,
J.M.Bostock,
W.Stubbings,
I.Chopra,
D.I.Roper,
A.J.Lloyd,
C.W.Fishwick,
and
A.P.Johnson
(2005).
A de novo designed inhibitor of D-Ala-D-Ala ligase from E. coli.
|
| |
Angew Chem Int Ed Engl,
44,
6403-6406.
|
 |
|
|
|
|
 |
J.Hiratake
(2005).
Enzyme inhibitors as chemical tools to study enzyme catalysis: rational design, synthesis, and applications.
|
| |
Chem Rec,
5,
209-228.
|
 |
|
|
|
|
 |
M.Sato,
K.Kirimura,
and
K.Kino
(2005).
D-Amino acid dipeptide production utilizing D-alanine-D-alanine ligases with novel substrate specificity.
|
| |
J Biosci Bioeng,
99,
623-628.
|
 |
|
|
|
|
 |
R.A.George,
R.V.Spriggs,
G.J.Bartlett,
A.Gutteridge,
M.W.MacArthur,
C.T.Porter,
B.Al-Lazikani,
J.M.Thornton,
and
M.B.Swindells
(2005).
Effective function annotation through catalytic residue conservation.
|
| |
Proc Natl Acad Sci U S A,
102,
12299-12304.
|
 |
|
|
|
|
 |
F.Depardieu,
M.Kolbert,
H.Pruul,
J.Bell,
and
P.Courvalin
(2004).
VanD-type vancomycin-resistant Enterococcus faecium and Enterococcus faecalis.
|
| |
Antimicrob Agents Chemother,
48,
3892-3904.
|
 |
|
|
|
|
 |
T.Hibi,
H.Nii,
T.Nakatsu,
A.Kimura,
H.Kato,
J.Hiratake,
and
J.Oda
(2004).
Crystal structure of gamma-glutamylcysteine synthetase: insights into the mechanism of catalysis by a key enzyme for glutathione homeostasis.
|
| |
Proc Natl Acad Sci U S A,
101,
15052-15057.
|
 |
|
PDB codes:
|
 |
|
|
|
|
|
 |
A.El Zoeiby,
F.Sanschagrin,
and
R.C.Levesque
(2003).
Structure and function of the Mur enzymes: development of novel inhibitors.
|
| |
Mol Microbiol,
47,
1.
|
 |
|
|
|
|
 |
F.Depardieu,
P.E.Reynolds,
and
P.Courvalin
(2003).
VanD-type vancomycin-resistant Enterococcus faecium 10/96A.
|
| |
Antimicrob Agents Chemother,
47,
7.
|
 |
|
|
|
|
 |
J.Pootoolal,
J.Neu,
and
G.D.Wright
(2002).
Glycopeptide antibiotic resistance.
|
| |
Annu Rev Pharmacol Toxicol,
42,
381-408.
|
 |
|
|
|
|
 |
O.H.Ambúr,
P.E.Reynolds,
and
C.A.Arias
(2002).
D-Ala:D-Ala ligase gene flanking the vanC cluster: evidence for presence of three ligase genes in vancomycin-resistant Enterococcus gallinarum BM4174.
|
| |
Antimicrob Agents Chemother,
46,
95.
|
 |
|
|
|
|
 |
S.Ramón-Maiques,
A.Marina,
F.Gil-Ortiz,
I.Fita,
and
V.Rubio
(2002).
Structure of acetylglutamate kinase, a key enzyme for arginine biosynthesis and a prototype for the amino acid kinase enzyme family, during catalysis.
|
| |
Structure,
10,
329-342.
|
 |
|
PDB codes:
|
 |
|
|
|
|
|
 |
D.C.Hooper
(2001).
Mechanisms of action of antimicrobials: focus on fluoroquinolones.
|
| |
Clin Infect Dis,
32,
S9.
|
 |
|
|
|
|
 |
G.D.Pullinger,
R.Sowdhamini,
and
A.J.Lax
(2001).
Localization of functional domains of the mitogenic toxin of Pasteurella multocida.
|
| |
Infect Immun,
69,
7839-7850.
|
 |
|
|
|
|
 |
K.A.Denessiouk,
V.V.Rantanen,
and
M.S.Johnson
(2001).
Adenine recognition: a motif present in ATP-, CoA-, NAD-, NADP-, and FAD-dependent proteins.
|
| |
Proteins,
44,
282-291.
|
 |
|
|
|
|
 |
Y.Gholizadeh,
M.Prevost,
F.Van Bambeke,
B.Casadewall,
P.M.Tulkens,
and
P.Courvalin
(2001).
Sequencing of the ddl gene and modeling of the mutated D-alanine:D-alanine ligase in glycopeptide-dependent strains of Enterococcus faecium.
|
| |
Protein Sci,
10,
836-844.
|
 |
|
|
|
|
 |
A.E.Belanger,
J.C.Porter,
and
G.F.Hatfull
(2000).
Genetic analysis of peptidoglycan biosynthesis in mycobacteria: characterization of a ddlA mutant of Mycobacterium smegmatis.
|
| |
J Bacteriol,
182,
6854-6856.
|
 |
|
|
|
|
 |
A.P.Kuzin,
T.Sun,
J.Jorczak-Baillass,
V.L.Healy,
C.T.Walsh,
and
J.R.Knox
(2000).
Enzymes of vancomycin resistance: the structure of D-alanine-D-lactate ligase of naturally resistant Leuconostoc mesenteroides.
|
| |
Structure,
8,
463-470.
|
 |
|
PDB code:
|
 |
|
|
|
|
|
 |
H.Berg,
K.Ziegler,
K.Piotukh,
K.Baier,
W.Lockau,
and
R.Volkmer-Engert
(2000).
Biosynthesis of the cyanobacterial reserve polymer multi-L-arginyl-poly-L-aspartic acid (cyanophycin): mechanism of the cyanophycin synthetase reaction studied with synthetic primers.
|
| |
Eur J Biochem,
267,
5561-5570.
|
 |
|
|
|
|
 |
J.B.Thoden,
S.Firestine,
A.Nixon,
S.J.Benkovic,
and
H.M.Holden
(2000).
Molecular structure of Escherichia coli PurT-encoded glycinamide ribonucleotide transformylase.
|
| |
Biochemistry,
39,
8791-8802.
|
 |
|
PDB codes:
|
 |
|
|
|
|
|
 |
K.A.Denessiouk,
and
M.S.Johnson
(2000).
When fold is not important: a common structural framework for adenine and AMP binding in 12 unrelated protein families.
|
| |
Proteins,
38,
310-326.
|
 |
|
|
|
|
 |
K.L.Levert,
R.B.Lloyd,
and
G.L.Waldrop
(2000).
Do cysteine 230 and lysine 238 of biotin carboxylase play a role in the activation of biotin?
|
| |
Biochemistry,
39,
4122-4128.
|
 |
|
|
|
|
 |
M.A.Joyce,
M.E.Fraser,
M.N.James,
W.A.Bridger,
and
W.T.Wolodko
(2000).
ADP-binding site of Escherichia coli succinyl-CoA synthetase revealed by x-ray crystallography.
|
| |
Biochemistry,
39,
17-25.
|
 |
|
PDB codes:
|
 |
|
|
|
|
|
 |
S.Ha,
D.Walker,
Y.Shi,
and
S.Walker
(2000).
The 1.9 A crystal structure of Escherichia coli MurG, a membrane-associated glycosyltransferase involved in peptidoglycan biosynthesis.
|
| |
Protein Sci,
9,
1045-1052.
|
 |
|
PDB code:
|
 |
|
|
|
|
|
 |
V.L.Healy,
I.A.Lessard,
D.I.Roper,
J.R.Knox,
and
C.T.Walsh
(2000).
Vancomycin resistance in enterococci: reprogramming of the D-ala-D-Ala ligases in bacterial peptidoglycan biosynthesis.
|
| |
Chem Biol,
7,
R109-R119.
|
 |
|
|
|
|
 |
V.L.Healy,
L.S.Mullins,
X.Li,
S.E.Hall,
F.M.Raushel,
and
C.T.Walsh
(2000).
D-Ala-D-X ligases: evaluation of D-alanyl phosphate intermediate by MIX, PIX and rapid quench studies.
|
| |
Chem Biol,
7,
505-514.
|
 |
|
|
|
|
 |
C.Li,
T.J.Kappock,
J.Stubbe,
T.M.Weaver,
and
S.E.Ealick
(1999).
X-ray crystal structure of aminoimidazole ribonucleotide synthetase (PurM), from the Escherichia coli purine biosynthetic pathway at 2.5 A resolution.
|
| |
Structure,
7,
1155-1166.
|
 |
|
PDB code:
|
 |
|
|
|
|
|
 |
F.Van Bambeke,
M.Chauvel,
P.E.Reynolds,
H.S.Fraimow,
and
P.Courvalin
(1999).
Vancomycin-dependent Enterococcus faecalis clinical isolates and revertant mutants.
|
| |
Antimicrob Agents Chemother,
43,
41-47.
|
 |
|
|
|
|
 |
G.Polekhina,
P.G.Board,
R.R.Gali,
J.Rossjohn,
and
M.W.Parker
(1999).
Molecular basis of glutathione synthetase deficiency and a rare gene permutation event.
|
| |
EMBO J,
18,
3204-3213.
|
 |
|
PDB code:
|
 |
|
|
|
|
|
 |
I.A.Lessard,
and
C.T.Walsh
(1999).
Mutational analysis of active-site residues of the enterococcal D-ala-D-Ala dipeptidase VanX and comparison with Escherichia coli D-ala-D-Ala ligase and D-ala-D-Ala carboxypeptidase VanY.
|
| |
Chem Biol,
6,
177-187.
|
 |
|
|
|
|
 |
J.B.Thoden,
F.M.Raushel,
M.M.Benning,
I.Rayment,
and
H.M.Holden
(1999).
The structure of carbamoyl phosphate synthetase determined to 2.1 A resolution.
|
| |
Acta Crystallogr D Biol Crystallogr,
55,
8.
|
 |
|
PDB code:
|
 |
|
|
|
|
|
 |
J.B.Thoden,
G.Wesenberg,
F.M.Raushel,
and
H.M.Holden
(1999).
Carbamoyl phosphate synthetase: closure of the B-domain as a result of nucleotide binding.
|
| |
Biochemistry,
38,
2347-2357.
|
 |
|
PDB code:
|
 |
|
|
|
|
|
 |
M.A.Joyce,
M.E.Fraser,
E.R.Brownie,
M.N.James,
W.A.Bridger,
and
W.T.Wolodko
(1999).
Probing the nucleotide-binding site of Escherichia coli succinyl-CoA synthetase.
|
| |
Biochemistry,
38,
7273-7283.
|
 |
|
|
|
|
 |
T.Huyton,
and
D.I.Roper
(1999).
Crystallization and preliminary X-ray characterization of VanA from Enterococcus faecium BM4147: towards the molecular basis of bacterial resistance to the glycopeptide antibiotic vancomycin.
|
| |
Acta Crystallogr D Biol Crystallogr,
55,
1481-1483.
|
 |
|
|
|
|
 |
T.M.Weaver,
W.Wang,
and
S.E.Ealick
(1999).
Purification, crystallization and preliminary X-ray diffraction data from selenomethionine glycinamide ribonucleotide synthetase.
|
| |
Acta Crystallogr D Biol Crystallogr,
55,
518-521.
|
 |
|
|
|
|
 |
Y.Yan,
S.Munshi,
Y.Li,
K.A.Pryor,
F.Marsilio,
and
B.Leiting
(1999).
Crystallization and preliminary X-ray analysis of the Escherichia coli UDP-MurNAc-tripeptide D-alanyl-D-alanine-adding enzyme (MurF).
|
| |
Acta Crystallogr D Biol Crystallogr,
55,
2033-2034.
|
 |
|
|
|
|
 |
C.G.Marshall,
and
G.D.Wright
(1998).
DdlN from vancomycin-producing Amycolatopsis orientalis C329.2 is a VanA homologue with D-alanyl-D-lactate ligase activity.
|
| |
J Bacteriol,
180,
5792-5795.
|
 |
|
|
|
|
 |
F.M.Raushel,
J.B.Thoden,
G.D.Reinhart,
and
H.M.Holden
(1998).
Carbamoyl phosphate synthetase: a crooked path from substrates to products.
|
| |
Curr Opin Chem Biol,
2,
624-632.
|
 |
|
|
|
|
 |
F.M.Raushel,
L.S.Mullins,
and
G.E.Gibson
(1998).
A stringent test for the nucleotide switch mechanism of carbamoyl phosphate synthetase.
|
| |
Biochemistry,
37,
10272-10278.
|
 |
|
|
|
|
 |
H.M.Holden,
J.B.Thoden,
and
F.M.Raushel
(1998).
Carbamoyl phosphate synthetase: a tunnel runs through it.
|
| |
Curr Opin Struct Biol,
8,
679-685.
|
 |
|
|
|
|
 |
K.A.Denessiouk,
J.V.Lehtonen,
and
M.S.Johnson
(1998).
Enzyme-mononucleotide interactions: three different folds share common structural elements for ATP recognition.
|
| |
Protein Sci,
7,
1768-1771.
|
 |
|
|
|
|
 |
K.A.Denessiouk,
J.V.Lehtonen,
T.Korpela,
and
M.S.Johnson
(1998).
Two "unrelated" families of ATP-dependent enzymes share extensive structural similarities about their cofactor binding sites.
|
| |
Protein Sci,
7,
1136-1146.
|
 |
|
|
|
|
 |
L.D.Gegnas,
S.T.Waddell,
R.M.Chabin,
S.Reddy,
and
K.K.Wong
(1998).
Inhibitors of the bacterial cell wall biosynthesis enzyme MurD.
|
| |
Bioorg Med Chem Lett,
8,
1643-1648.
|
 |
|
|
|
|
 |
L.Esser,
C.R.Wang,
M.Hosaka,
C.S.Smagula,
T.C.Südhof,
and
J.Deisenhofer
(1998).
Synapsin I is structurally similar to ATP-utilizing enzymes.
|
| |
EMBO J,
17,
977-984.
|
 |
|
PDB codes:
|
 |
|
|
|
|
|
 |
M.Arthur,
F.Depardieu,
L.Cabanié,
P.Reynolds,
and
P.Courvalin
(1998).
Requirement of the VanY and VanX D,D-peptidases for glycopeptide resistance in enterococci.
|
| |
Mol Microbiol,
30,
819-830.
|
 |
|
|
|
|
 |
M.McGuire,
K.Huang,
G.Kapadia,
O.Herzberg,
and
D.Dunaway-Mariano
(1998).
Location of the phosphate binding site within Clostridium symbiosum pyruvate phosphate dikinase.
|
| |
Biochemistry,
37,
13463-13474.
|
 |
|
PDB codes:
|
 |
|
|
|
|
|
 |
O.Dideberg,
and
J.Bertrand
(1998).
Tubulin tyrosine ligase: a shared fold with the glutathione synthetase ADP-forming family.
|
| |
Trends Biochem Sci,
23,
57-58.
|
 |
|
|
|
|
 |
V.L.Healy,
I.S.Park,
and
C.T.Walsh
(1998).
Active-site mutants of the VanC2 D-alanyl-D-serine ligase, characteristic of one vancomycin-resistant bacterial phenotype, revert towards wild-type D-alanyl-D-alanine ligases.
|
| |
Chem Biol,
5,
197-207.
|
 |
|
|
|
|
 |
V.M.Levdikov,
V.V.Barynin,
A.I.Grebenko,
W.R.Melik-Adamyan,
V.S.Lamzin,
and
K.S.Wilson
(1998).
The structure of SAICAR synthase: an enzyme in the de novo pathway of purine nucleotide biosynthesis.
|
| |
Structure,
6,
363-376.
|
 |
|
PDB code:
|
 |
|
|
|
|
|
 |
W.Wang,
T.J.Kappock,
J.Stubbe,
and
S.E.Ealick
(1998).
X-ray crystal structure of glycinamide ribonucleotide synthetase from Escherichia coli.
|
| |
Biochemistry,
37,
15647-15662.
|
 |
|
PDB code:
|
 |
|
|
|
|
|
 |
C.Fan,
I.S.Park,
C.T.Walsh,
and
J.R.Knox
(1997).
D-alanine:D-alanine ligase: phosphonate and phosphinate intermediates with wild type and the Y216F mutant.
|
| |
Biochemistry,
36,
2531-2538.
|
 |
|
PDB codes:
|
 |
|
|
|
|
|
 |
C.G.Marshall,
G.Broadhead,
B.K.Leskiw,
and
G.D.Wright
(1997).
D-Ala-D-Ala ligases from glycopeptide antibiotic-producing organisms are highly homologous to the enterococcal vancomycin-resistance ligases VanA and VanB.
|
| |
Proc Natl Acad Sci U S A,
94,
6480-6483.
|
 |
|
|
|
|
 |
I.S.Park,
C.H.Lin,
and
C.T.Walsh
(1997).
Bacterial resistance to vancomycin: overproduction, purification, and characterization of VanC2 from Enterococcus casseliflavus as a D-Ala-D-Ser ligase.
|
| |
Proc Natl Acad Sci U S A,
94,
10040-10044.
|
 |
|
|
|
|
 |
J.A.Bertrand,
G.Auger,
E.Fanchon,
L.Martin,
D.Blanot,
J.van Heijenoort,
and
O.Dideberg
(1997).
Crystal structure of UDP-N-acetylmuramoyl-L-alanine:D-glutamate ligase from Escherichia coli.
|
| |
EMBO J,
16,
3416-3425.
|
 |
|
PDB code:
|
 |
|
|
|
|
|
 |
J.B.Thoden,
H.M.Holden,
G.Wesenberg,
F.M.Raushel,
and
I.Rayment
(1997).
Structure of carbamoyl phosphate synthetase: a journey of 96 A from substrate to product.
|
| |
Biochemistry,
36,
6305-6316.
|
 |
|
|
|
|
 |
J.P.Shaw,
G.A.Petsko,
and
D.Ringe
(1997).
Determination of the structure of alanine racemase from Bacillus stearothermophilus at 1.9-A resolution.
|
| |
Biochemistry,
36,
1329-1342.
|
 |
|
PDB code:
|
 |
|
|
|
|
|
 |
M.Kothe,
B.Eroglu,
H.Mazza,
H.Samudera,
and
S.Powers-Lee
(1997).
Novel mechanism for carbamoyl-phosphate synthetase: a nucleotide switch for functionally equivalent domains.
|
| |
Proc Natl Acad Sci U S A,
94,
12348-12353.
|
 |
|
|
|
|
 |
M.Y.Galperin,
and
E.V.Koonin
(1997).
A diverse superfamily of enzymes with ATP-dependent carboxylate-amine/thiol ligase activity.
|
| |
Protein Sci,
6,
2639-2643.
|
 |
|
|
|
|
 |
N.Kobayashi,
and
N.Go
(1997).
ATP binding proteins with different folds share a common ATP-binding structural motif.
|
| |
Nat Struct Biol,
4,
6-7.
|
 |
|
|
|
|
 |
A.G.Murzin
(1996).
Structural classification of proteins: new superfamilies.
|
| |
Curr Opin Struct Biol,
6,
386-394.
|
 |
|
|
|
|
 |
A.M.Thunnissen,
and
B.W.Dijkstra
(1996).
Cure for a crisis?
|
| |
Nat Struct Biol,
3,
218-221.
|
 |
|
|
|
|
 |
B.A.Ellsworth,
N.J.Tom,
and
P.A.Bartlett
(1996).
Synthesis and evaluation of inhibitors of bacterial D-alanine:D-alanine ligases.
|
| |
Chem Biol,
3,
37-44.
|
 |
|
|
|
|
 |
C.T.Walsh,
S.L.Fisher,
I.S.Park,
M.Prahalad,
and
Z.Wu
(1996).
Bacterial resistance to vancomycin: five genes and one missing hydrogen bond tell the story.
|
| |
Chem Biol,
3,
21-28.
|
 |
|
|
|
|
 |
F.Javid-Majd,
M.A.Stapleton,
M.F.Harmon,
B.A.Hanks,
L.S.Mullins,
and
F.M.Raushel
(1996).
Comparison of the functional differences for the homologous residues within the carboxy phosphate and carbamate domains of carbamoyl phosphate synthetase.
|
| |
Biochemistry,
35,
14362-14369.
|
 |
|
|
|
|
 |
I.S.Park,
C.H.Lin,
and
C.T.Walsh
(1996).
Gain of D-alanyl-D-lactate or D-lactyl-D-alanine synthetase activities in three active-site mutants of the Escherichia coli D-alanyl-D-alanine ligase B.
|
| |
Biochemistry,
35,
10464-10471.
|
 |
|
|
|
|
 |
J.M.Ghuysen,
P.Charlier,
J.Coyette,
C.Duez,
E.Fonzé,
C.Fraipont,
C.Goffin,
B.Joris,
and
M.Nguyen-Distèche
(1996).
Penicillin and beyond: evolution, protein fold, multimodular polypeptides, and multiprotein complexes.
|
| |
Microb Drug Resist,
2,
163-175.
|
 |
|
|
|
|
 |
M.A.Stapleton,
F.Javid-Majd,
M.F.Harmon,
B.A.Hanks,
J.L.Grahmann,
L.S.Mullins,
and
F.M.Raushel
(1996).
Role of conserved residues within the carboxy phosphate domain of carbamoyl phosphate synthetase.
|
| |
Biochemistry,
35,
14352-14361.
|
 |
|
|
|
|
 |
M.McGuire,
L.J.Carroll,
L.Yankie,
S.H.Thrall,
D.Dunaway-Mariano,
O.Herzberg,
B.Jayaram,
and
B.H.Haley
(1996).
Determination of the nucleotide binding site within Clostridium symbiosum pyruvate phosphate dikinase by photoaffinity labeling, site-directed mutagenesis, and structural analysis.
|
| |
Biochemistry,
35,
8544-8552.
|
 |
|
|
|
|
 |
O.Herzberg,
C.C.Chen,
G.Kapadia,
M.McGuire,
L.J.Carroll,
S.J.Noh,
and
D.Dunaway-Mariano
(1996).
Swiveling-domain mechanism for enzymatic phosphotransfer between remote reaction sites.
|
| |
Proc Natl Acad Sci U S A,
93,
2652-2657.
|
 |
|
PDB code:
|
 |
|
|
|
|
|
 |
P.J.Artymiuk,
A.R.Poirrette,
D.W.Rice,
and
P.Willett
(1996).
Biotin carboxylase comes into the fold.
|
| |
Nat Struct Biol,
3,
128-132.
|
 |
|
|
|
|
 |
S.Evers,
B.Casadewall,
M.Charles,
S.Dutka-Malen,
M.Galimand,
and
P.Courvalin
(1996).
Evolution of structure and substrate specificity in D-alanine:D-alanine ligases and related enzymes.
|
| |
J Mol Evol,
42,
706-712.
|
 |
|
|
|
|
 |
T.Hara,
H.Kato,
Y.Katsube,
and
J.Oda
(1996).
A pseudo-michaelis quaternary complex in the reverse reaction of a ligase: structure of Escherichia coli B glutathione synthetase complexed with ADP, glutathione, and sulfate at 2.0 A resolution.
|
| |
Biochemistry,
35,
11967-11974.
|
 |
|
PDB code:
|
 |
|
|
|
|
|
 |
T.Skarzynski,
A.Mistry,
A.Wonacott,
S.E.Hutchinson,
V.A.Kelly,
and
K.Duncan
(1996).
Structure of UDP-N-acetylglucosamine enolpyruvyl transferase, an enzyme essential for the synthesis of bacterial peptidoglycan, complexed with substrate UDP-N-acetylglucosamine and the drug fosfomycin.
|
| |
Structure,
4,
1465-1474.
|
 |
|
PDB code:
|
 |
|
|
|
|
|
 |
C.Fan,
P.C.Moews,
Y.Shi,
C.T.Walsh,
and
J.R.Knox
(1995).
A common fold for peptide synthetases cleaving ATP to ADP: glutathione synthetase and D-alanine:d-alanine ligase of Escherichia coli.
|
| |
Proc Natl Acad Sci U S A,
92,
1172-1176.
|
 |
|
|
|
|
 |
D.Alexeev,
R.L.Baxter,
O.Smekal,
and
L.Sawyer
(1995).
Substrate binding and carboxylation by dethiobiotin synthetase--a kinetic and X-ray study.
|
| |
Structure,
3,
1207-1215.
|
 |
|
|
|
|
 |
R.C.Jackson
(1995).
Update on computer-aided drug design.
|
| |
Curr Opin Biotechnol,
6,
646-651.
|
 |
|
|
|
|
 |
Z.Wu,
and
C.T.Walsh
(1995).
Phosphinate analogs of D-, D-dipeptides: slow-binding inhibition and proteolysis protection of VanX, a D-, D-dipeptidase required for vancomycin resistance in Enterococcus faecium.
|
| |
Proc Natl Acad Sci U S A,
92,
11603-11607.
|
 |
|
 |
 |
|
The most recent references are shown first.
Citation data come partly from CiteXplore and partly
from an automated harvesting procedure. Note that this is likely to be
only a partial list as not all journals are covered by
either method. However, we are continually building up the citation data
so more and more references will be included with time.
Where a reference describes a PDB structure, the PDB
codes are
shown on the right.
|
 |

