 |
PDBsum entry 2d5c

|
|
|

|
 |

|

|

|

|

|

|

|

|

|

|

|

|

|

|

|

|

|

|
 |

|

|

|
 |
 |
 |
 |
 |
 |
 |
 |
 |
 |
 |
 |
 |
 |
 |
 |
 |
 |
 |
 |

|

|

|

|

|

|

|

|

|
|
Oxidoreductase
|
PDB id
|
|

|

|
2d5c
|

|

|

|

|

|

|

|

|

|

|

|

|

|

|

|

|

|

|

|
|
 |
Contents |
 |
|
|

|

|
|
|
|
|
|
|
|
* Residue conservation analysis
|
|
|
|
 |
|
|
 |
 |
 |
 |
Enzyme class:
|
 |
E.C.1.1.1.25
- shikimate dehydrogenase (NADP(+)).
|
|
 |
 |
 |
 |
 |

Pathway:
|
 |
Shikimate and Chorismate Biosynthesis
|
 |
 |
 |
 |
 |
Reaction:
|
 |
shikimate + NADP+ = 3-dehydroshikimate + NADPH + H+
|
 |
 |
 |
 |
 |
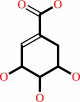 shikimate
shikimate
|
+
|
NADP(+)
Bound ligand (Het Group name = )
corresponds exactly
|
=
|
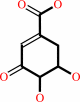 3-dehydroshikimate
3-dehydroshikimate
|
+
|
 NADPH
NADPH
|
+
|
H(+)
|
|
 |
 |
 |
 |
 |
 |
 |
 |
 |
 |
|
Molecule diagrams generated from .mol files obtained from the
KEGG ftp site
|
|
 |
 |
 |
 |
 |
 |
 |
 |
 |
 |
 |
 |
 |
 |
 |

|

|

|
| |
|
|
| |
|
DOI no:
|
J Mol Biol
373:424-438
(2007)
|
|
PubMed id:
|
|
|
|

|
| |
|
Crystal Structures of Shikimate Dehydrogenase AroE from Thermus thermophilus HB8 and its Cofactor and Substrate Complexes: Insights into the Enzymatic Mechanism.
|
|
B.Bagautdinov,
N.Kunishima.
|
|
|

|
| |
ABSTRACT
|
|

|
| |

|
Shikimate dehydrogenase (EC 1.1.1.25) catalyses the fourth step of the shikimate
pathway which is required for the synthesis of the aromatic amino acids and
other aromatic compounds in bacteria, microbial eukaryotes, and plants. The
crystal structures of the shikimate dehydrogenase AroE from Thermus thermophilus
HB8 in its ligand-free form, binary complexes with cofactor NADP(+) or substrate
shikimate, and the ternary complex with both NADP(H) and shikimate were
determined by X-ray diffraction method at atomic resolutions. The crystals are
nearly isomorphous with the asymmetric unit containing a dimer, each subunit of
which has a bi-domain structure of compact alpha/beta sandwich folds. The two
subunits of the enzyme display asymmetry in the crystals due to different
relative orientations between the N- and C-terminal domains resulting in a
slightly different closure of the interdomain clefts. NADP(H) is bound to the
more closed form only. This closed conformation with apparent higher affinity to
the cofactor is also observed in the unliganded crystal form, indicating that
the NADP(H) binding to TtAroE may follow the selection mode where the cofactor
binds to the subunit that happens to be in the closed conformation in solution.
Crystal structures of the closed subunits with and without NADP(H) show no
significant structural difference, suggesting that the cofactor binding to the
closed subunit corresponds to the lock-and-key model in TtAroE. On the other
hand, shikimate binds to both open and closed subunit conformers of both apo and
NADP(H)-liganded holo enzyme forms. The ternary complex TtAroE:NADP(H):shikimate
allows unambiguous visualization of the SDH permitting elucidation of the roles
of conserved residues Lys64 and Asp100 in the hydride ion transfer between
NADP(H) and shikimate.

|

|
|
|

|
| |
Selected figure(s)
|
|

|
| |
 |
 |

|
 |

|
 |
Figure 6.
Figure 6. Electrostatic potential surface of the ternary
complex. (a) Overall view. NADP(H) and shikimate molecules are
shown in stick models. Residues interacting with the
2′-phosphate group of cofactor are labeled. (b) Close-up view.
Catalytic residues and important ligand atoms are labeled.
|
 |
Figure 8.
Figure 8. A schematic representation of the proposed
catalytic mechanism of shikimate oxidation in TtAroE.
|
 |
|
|

|
| |
The above figures are
reprinted
by permission from Elsevier:
J Mol Biol
(2007,
373,
424-438)
copyright 2007.
|
|
| |
Figures were
selected
by an automated process.
|
|

|

|
|
 |
 |
|
 |
 |
 |
 |
 |
 |
 |
 |
 |
|
Literature references that cite this PDB file's key reference
|
|
 |
| |
PubMed id
|
 |
Reference
|
 |
|
|
|
 |
G.B.Barcellos,
R.A.Caceres,
and
W.F.de Azevedo
(2009).
Structural studies of shikimate dehydrogenase from Bacillus anthracis complexed with cofactor NADP.
|
| |
J Mol Model,
15,
147-155.
|
 |
|
|
|
|
 |
V.S.Rodrigues-Junior,
A.Breda,
D.S.Santos,
and
L.A.Basso
(2009).
The conserved Lysine69 residue plays a catalytic role in Mycobacterium tuberculosis shikimate dehydrogenase.
|
| |
BMC Res Notes,
2,
227.
|
 |
|
|
|
|
 |
J.Schoepe,
K.Niefind,
and
D.Schomburg
(2008).
1.6 A structure of an NAD(+)-dependent quinate dehydrogenase from Corynebacterium glutamicum.
|
| |
Acta Crystallogr D Biol Crystallogr,
64,
803-809.
|
 |
|
PDB code:
|
 |
|
|
|
|
|
 |
M.Sugahara,
Y.Asada,
K.Shimizu,
H.Yamamoto,
N.K.Lokanath,
H.Mizutani,
B.Bagautdinov,
Y.Matsuura,
M.Taketa,
Y.Kageyama,
N.Ono,
Y.Morikawa,
Y.Tanaka,
H.Shimada,
T.Nakamoto,
M.Sugahara,
M.Yamamoto,
and
N.Kunishima
(2008).
High-throughput crystallization-to-structure pipeline at RIKEN SPring-8 Center.
|
| |
J Struct Funct Genomics,
9,
21-28.
|
 |
|
 |
 |
|
The most recent references are shown first.
Citation data come partly from CiteXplore and partly
from an automated harvesting procedure. Note that this is likely to be
only a partial list as not all journals are covered by
either method. However, we are continually building up the citation data
so more and more references will be included with time.
Where a reference describes a PDB structure, the PDB
code is
shown on the right.
|
 |

