 |
PDBsum entry 2d1f

|

|

|
|
 |
Contents |
 |
|
|

|

|
|
|
|
|
|
|
|
* Residue conservation analysis
|
|
|

|
|
PDB id:
|
 |
|
 |
| Name: |
 |
Lyase
|
 |
|
Title:
|
 |
Structure of mycobacterium tuberculosis threonine synthase
|
|
Structure:
|
 |
Threonine synthase. Chain: a, b. Engineered: yes
|
|
Source:
|
 |
Mycobacterium tuberculosis. Organism_taxid: 1773. Expressed in: escherichia coli. Expression_system_taxid: 562
|
|
Biol. unit:
|
 |
Dimer (from
)
|
|
Resolution:
|
 |
|
2.50Å
|
R-factor:
|
0.191
|
R-free:
|
0.256
|
|
|
Authors:
|
 |
A.S.Covarrubias,T.Bergfors,K.Mannerstedt,S.Oscarson,T.A.Jones, S.L.Mowbray,M.Hogbom
|
Key ref:

|
 |
A.S.Covarrubias
et al.
(2008).
Structural, biochemical, and in vivo investigations of the threonine synthase from Mycobacterium tuberculosis.
J Mol Biol,
381,
622-633.
PubMed id:
DOI:

|
 |
|
Date:
|
 |
|
20-Aug-05
|
Release date:
|
05-Sep-06
|
|
|

|

|
|
PROCHECK
|

|

|
|
|
Headers
|
 |
|
|
References
|
|
|
|

|

|
|
P9WG59
(THRC_MYCTU) -
Threonine synthase from Mycobacterium tuberculosis (strain ATCC 25618 / H37Rv)
|
|

|

|
Seq:
Struc:
|
 |
 |
 |
360 a.a.
349 a.a.
|

|

|

|

|
|

|

|

|
 |
 |

|
|
Key: |
 |
PfamA domain |
 |
 |
 |
Secondary structure |
 |
 |
CATH domain |
 |
|

|
|
|
 |
|
|
 |
 |
 |
 |
Enzyme class:
|
 |
E.C.4.2.3.1
- threonine synthase.
|
|
 |
 |
 |
 |
 |

Pathway:
|
 |
Threonine Biosynthesis
|
 |
 |
 |
 |
 |
Reaction:
|
 |
O-phospho-L-homoserine + H2O = L-threonine + phosphate
|
 |
 |
 |
 |
 |
 O-phospho-L-homoserine
O-phospho-L-homoserine
|
+
|
H2O
|
=
|
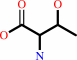 L-threonine
L-threonine
|
+
|
 phosphate
phosphate
|
|
 |
 |
 |
 |
 |
 |
 |
 |
Cofactor:
|
 |
Pyridoxal 5'-phosphate
|
 |
 |
 |
 |
 |
Pyridoxal 5'-phosphate
Bound ligand (Het Group name =
PLP)
matches with 93.75% similarity
|
|
 |
 |
 |
 |
|
Molecule diagrams generated from .mol files obtained from the
KEGG ftp site
|
|
 |
 |
 |
 |
 |
 |
 |
 |
 |
 |
 |
 |
 |
 |
 |

|

|

|
| |
|
|
| |
|
DOI no:
|
J Mol Biol
381:622-633
(2008)
|
|
PubMed id:
|
|
|
|

|
| |
|
Structural, biochemical, and in vivo investigations of the threonine synthase from Mycobacterium tuberculosis.
|
|
A.S.Covarrubias,
M.Högbom,
T.Bergfors,
P.Carroll,
K.Mannerstedt,
S.Oscarson,
T.Parish,
T.A.Jones,
S.L.Mowbray.
|
|
|

|
| |
ABSTRACT
|
|

|
| |

|
Threonine biosynthesis is a general feature of prokaryotes, eukaryotic
microorganisms, and higher plants. Since mammals lack the appropriate synthetic
machinery, instead obtaining the amino acid through their diet, the pathway is a
potential focus for the development of novel antibiotics, antifungal agents, and
herbicides. Threonine synthase (TS), a pyridoxal-5-phosphate-dependent enzyme,
catalyzes the final step in the pathway, in which L-homoserine phosphate and
water are converted into threonine and inorganic phosphate. In the present
publication, we report structural and functional studies of Mycobacterium
tuberculosis TS, the product of the rv1295 (thrC) gene. The structure gives new
insights into the catalytic mechanism of TSs in general, specifically by
suggesting the direct involvement of the phosphate moiety of the cofactor,
rather than the inorganic phosphate product, in transferring a proton from C4'
to C(gamma) in the formation of the alphabeta-unsaturated aldimine. It further
provides a basis for understanding why this enzyme has a higher pH optimum than
has been reported elsewhere for TSs and gives rise to the prediction that the
equivalent enzyme from Thermus thermophilus will exhibit similar behavior. A
deletion of the relevant gene generated a strain of M. tuberculosis that
requires threonine for growth; such auxotrophic strains are frequently
attenuated in vivo, indicating that TS is a potential drug target in this
organism.

|

|
|
|

|
| |
Selected figure(s)
|
|

|
| |
 |
 |

|
 |

|
 |
Figure 3.
Fig. 3. Comparison of MtTS and TtTS active sites. Active-site
residues of MtTS and the bound PLP are shown in gold, and
equivalent residues of TtTS are in gray. Two important hydrogen
bonds mentioned in the text are indicated by black dotted lines.
Electron density is for the PLP in the last 2|F[o]| − |F[c]|
map prior to its inclusion in the model, contoured at 1 σ = 0.2
e/Å^3.
|
 |
Figure 5.
Fig. 5. Features of particular interest in the TS catalytic
mechanism. A stereo view illustrates how the involvement of the
PLP 5′-phosphate group in the conversion of β,γ-unsaturated
ketimine to α,β-unsaturated aldimine is suggested by the
proximity of the relevant groups in the modeled reaction
intermediate, based on superposition of the Tt–AP5 structure
(shown by dotted lines). Residues that could influence the pH
optimum, as discussed in the text, are shown for MtTS (gold
carbons) and EcTS (cyan carbons).
|
 |
|
|

|
| |
The above figures are
reprinted
by permission from Elsevier:
J Mol Biol
(2008,
381,
622-633)
copyright 2008.
|
|
| |
Figures were
selected
by an automated process.
|
|

|

|
|
 |
 |
|
 |
 |
 |
 |
 |
 |
 |
 |
 |
|
Literature references that cite this PDB file's key reference
|
|
 |
| |
PubMed id
|
 |
Reference
|
 |
|
|
|
 |
D.E.Graham,
S.M.Taylor,
R.Z.Wolf,
and
S.C.Namboori
(2009).
Convergent evolution of coenzyme M biosynthesis in the Methanosarcinales: cysteate synthase evolved from an ancestral threonine synthase.
|
| |
Biochem J,
424,
467-478.
|
 |
|
 |
 |
|
The most recent references are shown first.
Citation data come partly from CiteXplore and partly
from an automated harvesting procedure. Note that this is likely to be
only a partial list as not all journals are covered by
either method. However, we are continually building up the citation data
so more and more references will be included with time.
|
 |

