
|

|

|
|
 |
Contents |
 |
|
|

|

|

|

|

|

|

|



 229 a.a.
229 a.a.
|
 |

|

|

|

|

|

|

|

 1119 a.a.
1119 a.a.
|
 |

|

|

|

|

|

|

|

 1392 a.a.
1392 a.a.
|
 |

|

|

|

|

|

|

|

 95 a.a.
95 a.a.
|
 |

|

|

|

|

|

|

|

 345 a.a.
345 a.a.
|
 |

|

|
|
|
|
|
|
|
* Residue conservation analysis
|
|
|

|
|
PDB id:
|
 |
|
 |
| Name: |
 |
Transferase
|
 |
|
Title:
|
 |
Crystal structure of the t. Thermophilus RNA polymerase holoenzyme
|
|
Structure:
|
 |
DNA-directed RNA polymerase alpha chain. Chain: a, b, k, l. Synonym: rnap alpha subunit, transcriptase alpha chain, RNA polymerase alpha subunit. DNA-directed RNA polymerase beta chain. Chain: c, m. Synonym: rnap beta subunit, transcriptase beta chain, RNA polymerase beta subunit. DNA-directed RNA polymerase beta' chain.
|
|
Source:
|
 |
Thermus thermophilus. Organism_taxid: 274. Organism_taxid: 274
|
|
Biol. unit:
|
 |
 Hexamer (from
)
Hexamer (from
)
|
|
Resolution:
|
 |
|
2.80Å
|
R-factor:
|
0.231
|
R-free:
|
0.268
|
|
|
Authors:
|
 |
I.Artsimovitch,M.N.Vassylyeva,D.Svetlov,V.Svetlov,A.Perederina, N.Igarashi,N.Matsugaki,S.Wakatsuki,T.H.Tahirov,D.G.Vassylyev,Riken Structural Genomics/proteomics Initiative (Rsgi)
|
Key ref:
|
 |
I.Artsimovitch
et al.
(2005).
Allosteric modulation of the RNA polymerase catalytic reaction is an essential component of transcription control by rifamycins.
Cell,
122,
351-363.
PubMed id:
DOI:

|
 |
|
Date:
|
 |
|
02-Jul-05
|
Release date:
|
20-Sep-05
|
|
|

|

|
|
PROCHECK
|

|

|
|
|
Headers
|
 |
|
|
References
|
|
|
|

|

|
|
Q5SHR6
(RPOA_THET8) -
DNA-directed RNA polymerase subunit alpha from Thermus thermophilus (strain ATCC 27634 / DSM 579 / HB8)
|
|

|

|
Seq:
Struc:
|
 |
 |
 |
315 a.a.
229 a.a.
|

|

|

|

|
|

|

|

|
 |
 |

|

|

|
|
Q8RQE9
(RPOB_THET8) -
DNA-directed RNA polymerase subunit beta from Thermus thermophilus (strain ATCC 27634 / DSM 579 / HB8)
|
|

|

|
Seq:
Struc:
|
 |
 |
 |
1119 a.a.
1119 a.a.
|

|

|

|

|
|

|

|

|
 |
 |

|

|

|
|
Q8RQE8
(RPOC_THET8) -
DNA-directed RNA polymerase subunit beta' from Thermus thermophilus (strain ATCC 27634 / DSM 579 / HB8)
|
|

|

|
Seq:
Struc:
|
 |
 |
 |
1524 a.a.
1392 a.a.
|

|

|

|

|
|

|

|

|
 |
 |

|
 |
|
|
 |
 |
 |
 |
Enzyme class:
|
 |
Chains A, B, C, D, E, K, L, M, N, O:
E.C.2.7.7.6
- DNA-directed Rna polymerase.
|
|
 |
 |
 |
 |
 |
Reaction:
|
 |
RNA(n) + a ribonucleoside 5'-triphosphate = RNA(n+1) + diphosphate
|
 |
 |
 |
 |
 |
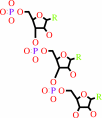 RNA(n)
RNA(n)
|
+
|
ribonucleoside 5'-triphosphate
|
=
|
RNA(n+1)
|
+
|
 diphosphate
diphosphate
|
|
 |
 |
 |
 |
 |
 |
 |
 |
 |
 |
|
Molecule diagrams generated from .mol files obtained from the
KEGG ftp site
|
|
 |
 |
 |
 |
 |
 |
 |
 |
 |
 |
 |
 |
 |
 |
 |

|

|

|
| |
|
|
| |
|
DOI no:
|
Cell
122:351-363
(2005)
|
|
PubMed id:
|
|
|
|

|
| |
|
Allosteric modulation of the RNA polymerase catalytic reaction is an essential component of transcription control by rifamycins.
|
|
I.Artsimovitch,
M.N.Vassylyeva,
D.Svetlov,
V.Svetlov,
A.Perederina,
N.Igarashi,
N.Matsugaki,
S.Wakatsuki,
T.H.Tahirov,
D.G.Vassylyev.
|
|
|

|
| |
ABSTRACT
|
|

|
| |

|
Rifamycins, the clinically important antibiotics, target bacterial RNA
polymerase (RNAP). A proposed mechanism in which rifamycins sterically block the
extension of nascent RNA beyond three nucleotides does not alone explain why
certain RNAP mutations confer resistance to some but not other rifamycins. Here
we show that unlike rifampicin and rifapentin, and contradictory to the steric
model, rifabutin inhibits formation of the first and second phosphodiester
bonds. We report 2.5 A resolution structures of rifabutin and rifapentin
complexed with the Thermus thermophilus RNAP holoenzyme. The structures reveal
functionally important distinct interactions of antibiotics with the initiation
sigma factor. Strikingly, both complexes lack the catalytic Mg2+ ion observed in
the apo-holoenzyme, whereas an increase in Mg2+ concentration confers resistance
to rifamycins. We propose that a rifamycin-induced signal is transmitted over
approximately 19 A to the RNAP active site to slow down catalysis. Based on
structural predictions, we designed enzyme substitutions that apparently
interrupt this allosteric signal.

|

|
|
|

|
| |
Selected figure(s)
|
|

|
| |
 |
 |

|
 |

|
 |
Figure 3.
Figure 3. RNAP/Rifs Binding
|
 |
Figure 5.
Figure 5. Increased Levels of Mg^2+ Ion Protect RNAP
against Inhibition by Rifs
|
 |
|
|

|
| |
The above figures are
reprinted
by permission from Cell Press:
Cell
(2005,
122,
351-363)
copyright 2005.
|
|
| |
Figures were
selected
by an automated process.
|
|

|

|
|
 |
 |
|
 |
 |
 |
 |
 |
 |
 |
 |
 |
|
Literature references that cite this PDB file's key reference
|
|
 |
| |
PubMed id
|
 |
Reference
|
 |
|
|
|
 |
G.K.Siu,
Y.Zhang,
T.C.Lau,
R.W.Lau,
P.L.Ho,
W.W.Yew,
S.K.Tsui,
V.C.Cheng,
K.Y.Yuen,
and
W.C.Yam
(2011).
Mutations outside the rifampicin resistance-determining region associated with rifampicin resistance in Mycobacterium tuberculosis.
|
| |
J Antimicrob Chemother,
66,
730-733.
|
 |
|
|
|
|
 |
A.Dey,
A.K.Verma,
and
D.Chatterji
(2010).
Role of an RNA polymerase interacting protein, MsRbpA, from Mycobacterium smegmatis in phenotypic tolerance to rifampicin.
|
| |
Microbiology,
156,
873-883.
|
 |
|
|
|
|
 |
A.Tupin,
M.Gualtieri,
J.P.Leonetti,
and
K.Brodolin
(2010).
The transcription inhibitor lipiarmycin blocks DNA fitting into the RNA polymerase catalytic site.
|
| |
EMBO J,
29,
2527-2537.
|
 |
|
|
|
|
 |
H.J.Yoon,
J.Kuwabara,
J.H.Kim,
and
C.A.Mirkin
(2010).
Allosteric supramolecular triple-layer catalysts.
|
| |
Science,
330,
66-69.
|
 |
|
|
|
|
 |
J.Cottreau,
S.F.Baker,
H.L.DuPont,
and
K.W.Garey
(2010).
Rifaximin: a nonsystemic rifamycin antibiotic for gastrointestinal infections.
|
| |
Expert Rev Anti Infect Ther,
8,
747-760.
|
 |
|
|
|
|
 |
M.K.Taha,
S.T.Hedberg,
M.Szatanik,
E.Hong,
C.Ruckly,
R.Abad,
S.Bertrand,
F.Carion,
H.Claus,
A.Corso,
R.Enríquez,
S.Heuberger,
W.Hryniewicz,
K.A.Jolley,
P.Kriz,
M.Mollerach,
M.Musilek,
A.Neri,
P.Olcén,
M.Pana,
A.Skoczynska,
C.Sorhouet Pereira,
P.Stefanelli,
G.Tzanakaki,
M.Unemo,
J.A.Vázquez,
U.Vogel,
and
I.Wasko
(2010).
Multicenter study for defining the breakpoint for rifampin resistance in Neisseria meningitidis by rpoB sequencing.
|
| |
Antimicrob Agents Chemother,
54,
3651-3658.
|
 |
|
|
|
|
 |
Y.Yuzenkova,
and
N.Zenkin
(2010).
Central role of the RNA polymerase trigger loop in intrinsic RNA hydrolysis.
|
| |
Proc Natl Acad Sci U S A,
107,
10878-10883.
|
 |
|
|
|
|
 |
D.G.Vassylyev
(2009).
Elongation by RNA polymerase: a race through roadblocks.
|
| |
Curr Opin Struct Biol,
19,
691-700.
|
 |
|
|
|
|
 |
E.Nudler
(2009).
RNA polymerase active center: the molecular engine of transcription.
|
| |
Annu Rev Biochem,
78,
335-361.
|
 |
|
|
|
|
 |
G.A.Belogurov,
M.N.Vassylyeva,
A.Sevostyanova,
J.R.Appleman,
A.X.Xiang,
R.Lira,
S.E.Webber,
S.Klyuyev,
E.Nudler,
I.Artsimovitch,
and
D.G.Vassylyev
(2009).
Transcription inactivation through local refolding of the RNA polymerase structure.
|
| |
Nature,
457,
332-335.
|
 |
|
PDB code:
|
 |
|
|
|
|
|
 |
I.Flåtten,
Morigen,
and
K.Skarstad
(2009).
DnaA protein interacts with RNA polymerase and partially protects it from the effect of rifampicin.
|
| |
Mol Microbiol,
71,
1018-1030.
|
 |
|
|
|
|
 |
M.X.Ho,
B.P.Hudson,
K.Das,
E.Arnold,
and
R.H.Ebright
(2009).
Structures of RNA polymerase-antibiotic complexes.
|
| |
Curr Opin Struct Biol,
19,
715-723.
|
 |
|
|
|
|
 |
A.Feklistov,
V.Mekler,
Q.Jiang,
L.F.Westblade,
H.Irschik,
R.Jansen,
A.Mustaev,
S.A.Darst,
and
R.H.Ebright
(2008).
Rifamycins do not function by allosteric modulation of binding of Mg2+ to the RNA polymerase active center.
|
| |
Proc Natl Acad Sci U S A,
105,
14820-14825.
|
 |
|
|
|
|
 |
F.Brueckner,
and
P.Cramer
(2008).
Structural basis of transcription inhibition by alpha-amanitin and implications for RNA polymerase II translocation.
|
| |
Nat Struct Mol Biol,
15,
811-818.
|
 |
|
PDB code:
|
 |
|
|
|
|
|
 |
G.T.Robertson,
E.J.Bonventre,
T.B.Doyle,
Q.Du,
L.Duncan,
T.W.Morris,
E.D.Roche,
D.Yan,
and
A.S.Lynch
(2008).
In vitro evaluation of CBR-2092, a novel rifamycin-quinolone hybrid antibiotic: studies of the mode of action in Staphylococcus aureus.
|
| |
Antimicrob Agents Chemother,
52,
2313-2323.
|
 |
|
|
|
|
 |
A.Szalewska-Palasz,
G.Wegrzyn,
and
A.Wegrzyn
(2007).
Mechanisms of physiological regulation of RNA synthesis in bacteria: new discoveries breaking old schemes.
|
| |
J Appl Genet,
48,
281-294.
|
 |
|
|
|
|
 |
V.Svetlov,
G.A.Belogurov,
E.Shabrova,
D.G.Vassylyev,
and
I.Artsimovitch
(2007).
Allosteric control of the RNA polymerase by the elongation factor RfaH.
|
| |
Nucleic Acids Res,
35,
5694-5705.
|
 |
|
|
|
|
 |
A.Kulbachinskiy,
and
A.Mustaev
(2006).
Region 3.2 of the sigma subunit contributes to the binding of the 3'-initiating nucleotide in the RNA polymerase active center and facilitates promoter clearance during initiation.
|
| |
J Biol Chem,
281,
18273-18276.
|
 |
|
|
|
|
 |
J.Davies,
G.B.Spiegelman,
and
G.Yim
(2006).
The world of subinhibitory antibiotic concentrations.
|
| |
Curr Opin Microbiol,
9,
445-453.
|
 |
|
|
|
|
 |
K.V.Newell,
D.P.Thomas,
D.Brekasis,
and
M.S.Paget
(2006).
The RNA polymerase-binding protein RbpA confers basal levels of rifampicin resistance on Streptomyces coelicolor.
|
| |
Mol Microbiol,
60,
687-696.
|
 |
|
|
|
|
 |
V.Trinh,
M.F.Langelier,
J.Archambault,
and
B.Coulombe
(2006).
Structural perspective on mutations affecting the function of multisubunit RNA polymerases.
|
| |
Microbiol Mol Biol Rev,
70,
12-36.
|
 |
|
|
|
|
 |
D.G.Vassylyev,
V.Svetlov,
M.N.Vassylyeva,
A.Perederina,
N.Igarashi,
N.Matsugaki,
S.Wakatsuki,
and
I.Artsimovitch
(2005).
Structural basis for transcription inhibition by tagetitoxin.
|
| |
Nat Struct Mol Biol,
12,
1086-1093.
|
 |
|
PDB code:
|
 |
|
|
 |
 |
|
The most recent references are shown first.
Citation data come partly from CiteXplore and partly
from an automated harvesting procedure. Note that this is likely to be
only a partial list as not all journals are covered by
either method. However, we are continually building up the citation data
so more and more references will be included with time.
Where a reference describes a PDB structure, the PDB
code is
shown on the right.
|

| |

