
|

|

|
|
 |
Contents |
 |
|
|

|

|
|
|
|
|
|
* Residue conservation analysis
|
|
|

|
|
PDB id:
|
 |
|
 |
| Name: |
 |
Transferase
|
 |
|
Title:
|
 |
Complex of enzyme iiamannose and the histidine-containing phosphocarrier protein hpr from escherichia coli nmr, restrained regularized mean structure
|
|
Structure:
|
 |
Pts system, mannose-specific iiab component. Chain: a, b. Fragment: eiia domain. Synonym: eiiab-man, mannose-permease iiab component, phosphotransferase enzyme ii, ab component, eiii-man. Engineered: yes. Phosphocarrier protein hpr. Chain: c, d. Synonym: histidine-containing protein.
|
|
Source:
|
 |
Escherichia coli. Organism_taxid: 562. Gene: manx, gptb, ptsl. Expressed in: escherichia coli. Expression_system_taxid: 562. Gene: ptsh, hpr. Expression_system_taxid: 562
|
|
NMR struc:
|
 |
2 models
|
 |
|
Authors:
|
 |
G.M.Clore,D.C.Williams
|
Key ref:

|
 |
D.C.Williams
et al.
(2005).
Solution NMR structure of the 48-kDa IIAMannose-HPr complex of the Escherichia coli mannose phosphotransferase system.
J Biol Chem,
280,
20775-20784.
PubMed id:
DOI:

|
 |
|
Date:
|
 |
|
21-Feb-05
|
Release date:
|
19-Apr-05
|
|
|

|

|
|
PROCHECK
|

|

|
|
|
Headers
|
 |
|
|
References
|
|
|
|
|
 |
|
|
 |
 |
 |
 |
Enzyme class:
|
 |
Chains A, B:
E.C.2.7.1.191
- protein-N(pi)-phosphohistidine--D-mannose phosphotransferase.
|
|
 |
 |
 |
 |
 |
Reaction:
|
 |
D-mannose(out) + N(pros)-phospho-L-histidyl-[protein] = D-mannose 6-phosphate(in) + L-histidyl-[protein]
|
 |
 |
 |
 |
 |
[Protein]-N(pi)-phospho-L-histidine
|
+
|
D-mannose(Side 1)
|
=
|
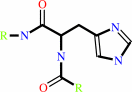 [protein]-L- histidine
[protein]-L- histidine
|
+
|
D-mannose 6-phosphate(Side 2)
|
|
 |
 |
 |
 |
 |
 |
 |
 |
 |
 |
|
Molecule diagrams generated from .mol files obtained from the
KEGG ftp site
|
|
 |
 |
 |
 |
 |
 |
 |
 |
 |
 |
 |
 |
 |
 |
 |

|

|

|
| |
|
|
| |
|
DOI no:
|
J Biol Chem
280:20775-20784
(2005)
|
|
PubMed id:
|
|
|
|

|
| |
|
Solution NMR structure of the 48-kDa IIAMannose-HPr complex of the Escherichia coli mannose phosphotransferase system.
|
|
D.C.Williams,
M.Cai,
J.Y.Suh,
A.Peterkofsky,
G.M.Clore.
|
|
|

|
| |
ABSTRACT
|
|

|
| |

|
The solution structure of the 48-kDa IIA(Man)-HPr complex of the mannose branch
of the Escherichia coli phosphotransferase system has been solved by NMR using
conjoined rigid body/torsion angle-simulated annealing on the basis of
intermolecular nuclear Overhauser enhancement data and residual dipolar
couplings. IIA(Man) is dimeric and has two symmetrically related binding sites
per dimer for HPr. A convex surface on HPr, formed primarily by helices 1 and 2,
interacts with a deep groove at the interface of the two subunits of IIA(Man).
The interaction surface on IIA(Man) is predominantly helical, comprising helix 3
from the subunit that bears the active site His-10 and helices 1, 4, and 5 from
the other subunit. The total buried accessible surface area at the
protein-protein interface is 1450 A(2). The binding sites on the two proteins
are complementary in terms of shape and distribution of hydrophobic,
hydrophilic, and charged residues. The active site histidines, His-10 of
IIA(Man) and His-15 (italics indicate HPr residues) of HPr, are in close
proximity. An associative transition state involving a pentacoordinate
phosphoryl group with trigonal bipyramidal geometry bonded to the N-epsilon2
atom of His-10 and the N-delta1 atom of His-15 can be readily formed with
negligible displacement in the backbone coordinates of the residues immediately
adjacent to the active site histidines. Comparing the structures of complexes of
HPr with three other structurally unrelated phosphotransferase system proteins,
enzymes I, IIA(glucose), and IIA(mannitol), reveals a number of common features
that provide a molecular basis for understanding how HPr specifically recognizes
a wide range of diverse proteins.

|

|
|
|

|
| |
Selected figure(s)
|
|

|
| |
 |
 |

|
 |

|
 |
Figure 5.
FIG. 5. The IIA^Man-HPr interface. A, stereoview of the
interface with the backbone shown as tubes (green, HPr; blue,
subunit A of IIA^Man; red, subunit B of IIA^Man) and the side
chains as bonds (light green, HPr; cyan, subunit A of IIA^Man;
pink, subunit B of IIA^Man; purple, active site histidines).
Residues of HPr are labeled in italics. Note that the  1-ppm
chemical shift perturbation observed for the backbone amide of
Ser-72 of IIA^Man (cf. Fig. 1) is because of a ring current
shift arising from its close proximity ( 1-ppm
chemical shift perturbation observed for the backbone amide of
Ser-72 of IIA^Man (cf. Fig. 1) is because of a ring current
shift arising from its close proximity (  3.9 Å) to the
imidazole ring of His-15 of HPr. No other backbone amide proton
of IIA^Man is within 5.7 Å of an aromatic side chain of
HPr. B, diagrammatic summary of interfacial contacts.
Residuesinvolved in potential electrostatic, hydrogen-bonding,
or water-mediated hydrogen-bonding interactions are indicated in
red (side chain-side chain contacts), blue (side chain of HPr to
backbone carbonyl of IIA^Man), or green (side chain of IIA^Man
to backbone carbonyl of HPr). The active site histidines are
shown in purple. C, interaction surface on IIA^Man for binding
HPr. D, interaction surface on HPr for binding IIA^Man. Residues
in the interaction surfaces (C and D) are color-coded as
hydrophobic (green), hydrophilic (cyan), positively charged
(blue), or negatively charged (red). The relevant portions of
the backbone of the interacting partner, HPr in C (gold) and
IIA^Man in D (lilac for subunit A, gold for subunit B,) are
displayed as tubes. In C, the surface of the non-interacting
residues of IIA^Man is colored in dark gray for subunit A and
light gray for subunit B. Residues of HPr are labeled in italics. 3.9 Å) to the
imidazole ring of His-15 of HPr. No other backbone amide proton
of IIA^Man is within 5.7 Å of an aromatic side chain of
HPr. B, diagrammatic summary of interfacial contacts.
Residuesinvolved in potential electrostatic, hydrogen-bonding,
or water-mediated hydrogen-bonding interactions are indicated in
red (side chain-side chain contacts), blue (side chain of HPr to
backbone carbonyl of IIA^Man), or green (side chain of IIA^Man
to backbone carbonyl of HPr). The active site histidines are
shown in purple. C, interaction surface on IIA^Man for binding
HPr. D, interaction surface on HPr for binding IIA^Man. Residues
in the interaction surfaces (C and D) are color-coded as
hydrophobic (green), hydrophilic (cyan), positively charged
(blue), or negatively charged (red). The relevant portions of
the backbone of the interacting partner, HPr in C (gold) and
IIA^Man in D (lilac for subunit A, gold for subunit B,) are
displayed as tubes. In C, the surface of the non-interacting
residues of IIA^Man is colored in dark gray for subunit A and
light gray for subunit B. Residues of HPr are labeled in italics.
|
 |
Figure 6.
FIG. 6. Comparison of the relative orientation of the
interacting helices in the IIA^Man-HPr and EIN-HPr complexes.
The two complexes have been best-fitted to the coordinates of
HPr. Two approximately orthogonal views are shown with the
helices of HPr (  1 and 1 and  2) in
green; helices 2) in
green; helices  1, 1,  4, and 4, and  5 of
subunit B of IIA^Man in red; helix 5 of
subunit B of IIA^Man in red; helix  3 of subunit A of
IIA^Man in blue; and helices 3 of subunit A of
IIA^Man in blue; and helices  2, 2,  2', 2',  3, and 3, and  4 of EIN
in gold. The coordinates of the EIN-HPr complex are taken from
Ref. 5 (Protein Data Bank accession code 3EZA [PDB]
). 4 of EIN
in gold. The coordinates of the EIN-HPr complex are taken from
Ref. 5 (Protein Data Bank accession code 3EZA [PDB]
).
|
 |
|
|

|
| |
The above figures are
reprinted
from an Open Access publication published by the ASBMB:
J Biol Chem
(2005,
280,
20775-20784)
copyright 2005.
|
|
| |
Figures were
selected
by an automated process.
|
|

|

|
|
 |
 |
|
 |
 |
 |
 |
 |
 |
 |
 |
 |
|
Literature references that cite this PDB file's key reference
|
|
 |
| |
PubMed id
|
 |
Reference
|
 |
|
|
|
 |
V.Roldós,
F.J.Cañada,
and
J.Jiménez-Barbero
(2011).
Carbohydrate-protein interactions: a 3D view by NMR.
|
| |
Chembiochem,
12,
990.
|
 |
|
|
|
|
 |
F.Ferrage,
K.Dutta,
A.Shekhtman,
and
D.Cowburn
(2010).
Structural determination of biomolecular interfaces by nuclear magnetic resonance of proteins with reduced proton density.
|
| |
J Biomol NMR,
47,
41-54.
|
 |
|
|
|
|
 |
Y.S.Jung,
M.Cai,
and
G.M.Clore
(2010).
Solution structure of the IIAChitobiose-IIBChitobiose complex of the N,N'-diacetylchitobiose branch of the Escherichia coli phosphotransferase system.
|
| |
J Biol Chem,
285,
4173-4184.
|
 |
|
PDB codes:
|
 |
|
|
|
|
|
 |
G.M.Clore,
and
J.Iwahara
(2009).
Theory, practice, and applications of paramagnetic relaxation enhancement for the characterization of transient low-population states of biological macromolecules and their complexes.
|
| |
Chem Rev,
109,
4108-4139.
|
 |
|
|
|
|
 |
D.J.Roy,
I.Casabon,
K.Vaillancourt,
J.L.Huot,
and
C.Vadeboncoeur
(2008).
Streptococci and lactococci synthesize large amounts of HPr(Ser-P)(His~P).
|
| |
Can J Microbiol,
54,
941-949.
|
 |
|
|
|
|
 |
J.Hu,
K.Hu,
D.C.Williams,
M.E.Komlosh,
M.Cai,
and
G.M.Clore
(2008).
Solution NMR structures of productive and non-productive complexes between the A and B domains of the cytoplasmic subunit of the mannose transporter of the Escherichia coli phosphotransferase system.
|
| |
J Biol Chem,
283,
11024-11037.
|
 |
|
PDB codes:
|
 |
|
|
|
|
|
 |
J.Y.Suh,
M.Cai,
and
G.M.Clore
(2008).
Impact of phosphorylation on structure and thermodynamics of the interaction between the N-terminal domain of enzyme I and the histidine phosphocarrier protein of the bacterial phosphotransferase system.
|
| |
J Biol Chem,
283,
18980-18989.
|
 |
|
|
|
|
 |
P.R.Markwick,
T.Malliavin,
and
M.Nilges
(2008).
Structural biology by NMR: structure, dynamics, and interactions.
|
| |
PLoS Comput Biol,
4,
e1000168.
|
 |
|
|
|
|
 |
Y.C.Kim,
C.Tang,
G.M.Clore,
and
G.Hummer
(2008).
Replica exchange simulations of transient encounter complexes in protein-protein association.
|
| |
Proc Natl Acad Sci U S A,
105,
12855-12860.
|
 |
|
|
|
|
 |
B.Reichenbach,
D.A.Breustedt,
J.Stülke,
B.Rak,
and
B.Görke
(2007).
Genetic dissection of specificity determinants in the interaction of HPr with enzymes II of the bacterial phosphoenolpyruvate:sugar phosphotransferase system in Escherichia coli.
|
| |
J Bacteriol,
189,
4603-4613.
|
 |
|
|
|
|
 |
J.Y.Suh,
J.Iwahara,
and
G.M.Clore
(2007).
Intramolecular domain-domain association/dissociation and phosphoryl transfer in the mannitol transporter of Escherichia coli are not coupled.
|
| |
Proc Natl Acad Sci U S A,
104,
3153-3158.
|
 |
|
|
|
|
 |
W.Müller,
and
H.Sticht
(2007).
A protein-specifically adapted scoring function for the reranking of docking solutions.
|
| |
Proteins,
67,
98.
|
 |
|
|
|
|
 |
C.Tang,
and
G.M.Clore
(2006).
A simple and reliable approach to docking protein-protein complexes from very sparse NOE-derived intermolecular distance restraints.
|
| |
J Biomol NMR,
36,
37-44.
|
 |
|
|
|
|
 |
C.Tang,
J.Iwahara,
and
G.M.Clore
(2006).
Visualization of transient encounter complexes in protein-protein association.
|
| |
Nature,
444,
383-386.
|
 |
|
|
|
|
 |
J.Deutscher,
C.Francke,
and
P.W.Postma
(2006).
How phosphotransferase system-related protein phosphorylation regulates carbohydrate metabolism in bacteria.
|
| |
Microbiol Mol Biol Rev,
70,
939.
|
 |
|
|
|
|
 |
J.Vaynberg,
and
J.Qin
(2006).
Weak protein-protein interactions as probed by NMR spectroscopy.
|
| |
Trends Biotechnol,
24,
22-27.
|
 |
|
|
|
|
 |
K.Hu,
B.Vögeli,
and
G.M.Clore
(2006).
13C-detected HN(CA)C and HMCMC experiments using a single methyl-reprotonated sample for unambiguous methyl resonance assignment.
|
| |
J Biomol NMR,
36,
259-266.
|
 |
|
|
|
|
 |
A.Bax,
and
A.Grishaev
(2005).
Weak alignment NMR: a hawk-eyed view of biomolecular structure.
|
| |
Curr Opin Struct Biol,
15,
563-570.
|
 |
|
|
|
|
 |
A.M.Bonvin,
R.Boelens,
and
R.Kaptein
(2005).
NMR analysis of protein interactions.
|
| |
Curr Opin Chem Biol,
9,
501-508.
|
 |
|
 |
 |
|
The most recent references are shown first.
Citation data come partly from CiteXplore and partly
from an automated harvesting procedure. Note that this is likely to be
only a partial list as not all journals are covered by
either method. However, we are continually building up the citation data
so more and more references will be included with time.
Where a reference describes a PDB structure, the PDB
codes are
shown on the right.
|
 |

