 |
PDBsum entry 1vnc

|
|
|

|
 |

|

|

|

|

|

|

|

|

|

|

|

|

|

|

|

|
 |

|

|

|
 |
 |
 |
 |
 |
 |
 |
 |
 |
 |
 |
 |
 |
 |
 |
 |
 |
 |
 |

|

|

|

|

|

|

|

|

|
|
Oxidoreductase
|
PDB id
|
|

|

|
1vnc
|

|

|

|

|

|

|

|

|

|

|

|

|

|

|

|

|

|

|

|
|
 |
Contents |
 |
|
|

|

|
|
|
|
|
|
|
|
* Residue conservation analysis
|
|
|
|
 |
|
|
 |
 |
 |
 |
Enzyme class:
|
 |
E.C.1.11.1.10
- chloride peroxidase.
|
|
 |
 |
 |
 |
 |
Reaction:
|
 |
RH + Cl- + H2O2 = RCl + 2 H2O
|
 |
 |
 |
 |
 |
RH
|
+
|
Cl(-)
|
+
|
 H2O2
H2O2
|
=
|
 RCl
RCl
|
+
|
2
×
H2O
|
|
 |
 |
 |
 |
 |
 |
 |
 |
Cofactor:
|
 |
Heme
|
 |
 |
 |
 |
 |
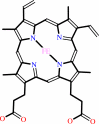 Heme
Heme
|
|
 |
 |
 |
 |
|
Molecule diagrams generated from .mol files obtained from the
KEGG ftp site
|
|
 |
 |
 |
 |
 |
 |
 |
 |
 |
 |
 |
 |
 |
 |
 |

|

|

|
| |
|
|
| |
|
|
Proc Natl Acad Sci U S A
93:392-396
(1996)
|
|
PubMed id:
|
|
|
|

|
| |
|
X-ray structure of a vanadium-containing enzyme: chloroperoxidase from the fungus Curvularia inaequalis.
|
|
A.Messerschmidt,
R.Wever.
|
|
|

|
| |
ABSTRACT
|
|

|
| |

|
The chloroperoxidase (EC 1.11.1.-) from the fungus Curvularia inaequalis belongs
to a class of vanadium enzymes that oxidize halides in the presence of hydrogen
peroxide to the corresponding hypohalous acids. The 2.1 A crystal structure (R =
20%) of an azide chloroperoxidase complex reveals the geometry of the catalytic
vanadium center. Azide coordinates directly to the metal center, resulting in a
structure with azide, three nonprotein oxygens, and a histidine as ligands. In
the native state vanadium will be bound as hydrogen vanadate(V) in a trigonal
bipyramidal coordination with the metal coordinated to three oxygens in the
equatorial plane, to the OH group at one apical position, and to the epsilon 2
nitrogen of a histidine at the other apical position. The protein fold is mainly
alpha-helical with two four-helix bundles as main structural motifs and an
overall structure different from other structures. The helices pack together to
a compact molecule, which explains the high stability of the protein. An amino
acid sequence comparison with vanadium-containing bromoperoxidase from the
seaweed Ascophyllum nodosum shows high similarities in the regions of the metal
binding site, with all hydrogen vanadate(V) interacting residues conserved
except for lysine-353, which is an asparagine.

|

|
|
|

|

|
|
 |
 |
|
 |
 |
 |
 |
 |
 |
 |
 |
 |
|
Literature references that cite this PDB file's key reference
|
|
 |
| |
PubMed id
|
 |
Reference
|
 |
|
|
|
 |
F.Manabe,
H.Shoun,
and
T.Wakagi
(2011).
Conserved residues in membrane-bound acid pyrophosphatase from Sulfolobus tokodaii, a thermoacidophilic archaeon.
|
| |
Extremophiles,
15,
359-364.
|
 |
|
|
|
|
 |
M.R.Maurya,
A.A.Khan,
A.Azam,
S.Ranjan,
N.Mondal,
A.Kumar,
F.Avecilla,
and
J.C.Pessoa
(2010).
Vanadium complexes having [V(IV)O](2+) and [V(V)O(2)](+) cores with binucleating dibasic tetradentate ligands: Synthesis, characterization, catalytic and antiamoebic activities.
|
| |
Dalton Trans,
39,
1345-1360.
|
 |
|
|
|
|
 |
A.Butler,
and
M.Sandy
(2009).
Mechanistic considerations of halogenating enzymes.
|
| |
Nature,
460,
848-854.
|
 |
|
|
|
|
 |
J.M.Winter,
and
B.S.Moore
(2009).
Exploring the chemistry and biology of vanadium-dependent haloperoxidases.
|
| |
J Biol Chem,
284,
18577-18581.
|
 |
|
|
|
|
 |
K.Kikushima,
T.Moriuchi,
and
T.Hirao
(2009).
Vanadium-catalyzed oxidative bromination under atmospheric oxygen.
|
| |
Chem Asian J,
4,
1213-1216.
|
 |
|
|
|
|
 |
Y.Chen,
J.Jakoncic,
K.A.Parker,
N.Carpino,
and
N.Nassar
(2009).
Structures of the phosphorylated and VO(3)-bound 2H-phosphatase domain of Sts-2.
|
| |
Biochemistry,
48,
8129-8135.
|
 |
|
|
|
|
 |
C.S.Neumann,
D.G.Fujimori,
and
C.T.Walsh
(2008).
Halogenation strategies in natural product biosynthesis.
|
| |
Chem Biol,
15,
99.
|
 |
|
|
|
|
 |
R.A.Sheldon
(2008).
E factors, green chemistry and catalysis: an odyssey.
|
| |
Chem Commun (Camb),
(),
3352-3365.
|
 |
|
|
|
|
 |
G.Moro,
L.Bonati,
M.Bruschi,
U.Cosentino,
L.De Gioia,
P.C.Fantucci,
A.Pandini,
E.Papaleo,
D.Pitea,
G.A.Saracino,
and
G.Zampella
(2007).
Computational approaches to shed light on molecular mechanisms in biological processes.
|
| |
Theor Chem Acc,
117,
723-741.
|
 |
|
|
|
|
 |
J.P.Bellenger,
F.Arnaud-Neu,
Z.Asfari,
S.C.Myneni,
E.I.Stiefel,
and
A.M.Kraepiel
(2007).
Complexation of oxoanions and cationic metals by the biscatecholate siderophore azotochelin.
|
| |
J Biol Inorg Chem,
12,
367-376.
|
 |
|
|
|
|
 |
C.Letondor,
and
T.R.Ward
(2006).
Artificial metalloenzymes for enantioselective catalysis: recent advances.
|
| |
Chembiochem,
7,
1845-1852.
|
 |
|
|
|
|
 |
M.R.Maurya,
S.Agarwal,
M.Abid,
A.Azam,
C.Bader,
M.Ebel,
and
D.Rehder
(2006).
Synthesis, characterisation, reactivity and in vitro antiamoebic activity of hydrazone based oxovanadium(IV), oxovanadium(V) and mu-bis(oxo)bis{oxovanadium(V)} complexes.
|
| |
Dalton Trans,
(),
937-947.
|
 |
|
|
|
|
 |
M.R.Maurya,
U.Kumar,
and
P.Manikandan
(2006).
Polymer supported vanadium and molybdenum complexes as potential catalysts for the oxidation and oxidative bromination of organic substrates.
|
| |
Dalton Trans,
(),
3561-3575.
|
 |
|
|
|
|
 |
C.Colin,
C.Leblanc,
G.Michel,
E.Wagner,
E.Leize-Wagner,
A.Van Dorsselaer,
and
P.Potin
(2005).
Vanadium-dependent iodoperoxidases in Laminaria digitata, a novel biochemical function diverging from brown algal bromoperoxidases.
|
| |
J Biol Inorg Chem,
10,
156-166.
|
 |
|
|
|
|
 |
E.Garcia-Rodriguez,
T.Ohshiro,
T.Aibara,
Y.Izumi,
and
J.Littlechild
(2005).
Enhancing effect of calcium and vanadium ions on thermal stability of bromoperoxidase from Corallina pilulifera.
|
| |
J Biol Inorg Chem,
10,
275-282.
|
 |
|
PDB code:
|
 |
|
|
|
|
|
 |
M.R.Maurya,
S.Agarwal,
C.Bader,
M.Ebel,
and
D.Rehder
(2005).
Synthesis, characterisation and catalytic potential of hydrazonato-vanadium(V) model complexes with [VO]3+ and [VO2]+ cores.
|
| |
Dalton Trans,
(),
537-544.
|
 |
|
|
|
|
 |
S.Pyne,
K.C.Kong,
and
P.I.Darroch
(2004).
Lysophosphatidic acid and sphingosine 1-phosphate biology: the role of lipid phosphate phosphatases.
|
| |
Semin Cell Dev Biol,
15,
491-501.
|
 |
|
|
|
|
 |
T.Ohshiro,
J.Littlechild,
E.Garcia-Rodriguez,
M.N.Isupov,
Y.Iida,
T.Kobayashi,
and
Y.Izumi
(2004).
Modification of halogen specificity of a vanadium-dependent bromoperoxidase.
|
| |
Protein Sci,
13,
1566-1571.
|
 |
|
|
|
|
 |
A.Kihara,
T.Sano,
S.Iwaki,
and
Y.Igarashi
(2003).
Transmembrane topology of sphingoid long-chain base-1-phosphate phosphatase, Lcb3p.
|
| |
Genes Cells,
8,
525-535.
|
 |
|
|
|
|
 |
G.Tigyi,
and
A.L.Parrill
(2003).
Molecular mechanisms of lysophosphatidic acid action.
|
| |
Prog Lipid Res,
42,
498-526.
|
 |
|
|
|
|
 |
J.Littlechild,
E.Garcia-Rodriguez,
A.Dalby,
and
M.Isupov
(2002).
Structural and functional comparisons between vanadium haloperoxidase and acid phosphatase enzymes.
|
| |
J Mol Recognit,
15,
291-296.
|
 |
|
|
|
|
 |
N.Bar-Nun,
S.Shcolnick,
and
A.M.Mayer
(2002).
Presence of a vanadium-dependent haloperoxidase in Botrytis cinerea.
|
| |
FEMS Microbiol Lett,
217,
121-124.
|
 |
|
|
|
|
 |
N.Tanaka,
V.Dumay,
Q.Liao,
A.J.Lange,
and
R.Wever
(2002).
Bromoperoxidase activity of vanadate-substituted acid phosphatases from Shigella flexneri and Salmonella enterica ser. typhimurium.
|
| |
Eur J Biochem,
269,
2162-2167.
|
 |
|
|
|
|
 |
F.van de Velde,
F.van Rantwijk,
and
R.A.Sheldon
(2001).
Improving the catalytic performance of peroxidases in organic synthesis.
|
| |
Trends Biotechnol,
19,
73-80.
|
 |
|
|
|
|
 |
H.B.ten Brink,
H.E.Schoemaker,
and
R.Wever
(2001).
Sulfoxidation mechanism of vanadium bromoperoxidase from Ascophyllum nodosum. Evidence for direct oxygen transfer catalysis.
|
| |
Eur J Biochem,
268,
132-138.
|
 |
|
|
|
|
 |
H.Schmidt,
I.Andersson,
D.Rehder,
and
L.Pettersson
(2001).
A potentiometric and 51V NMR study of the aqueous H+/H2VO4-/H2O2/L-alpha-alanyl-L-histidine system.
|
| |
Chemistry,
7,
251-257.
|
 |
|
|
|
|
 |
U.Dengler,
A.S.Siddiqui,
and
G.J.Barton
(2001).
Protein structural domains: analysis of the 3Dee domains database.
|
| |
Proteins,
42,
332-344.
|
 |
|
|
|
|
 |
G.van de Werve,
A.Lange,
C.Newgard,
M.C.Méchin,
Y.Li,
and
A.Berteloot
(2000).
New lessons in the regulation of glucose metabolism taught by the glucose 6-phosphatase system.
|
| |
Eur J Biochem,
267,
1533-1549.
|
 |
|
|
|
|
 |
K.Ishikawa,
Y.Mihara,
K.Gondoh,
E.Suzuki,
and
Y.Asano
(2000).
X-ray structures of a novel acid phosphatase from Escherichia blattae and its complex with the transition-state analog molybdate.
|
| |
EMBO J,
19,
2412-2423.
|
 |
|
PDB codes:
|
 |
|
|
|
|
|
 |
D.A.Toke,
M.L.McClintick,
and
G.M.Carman
(1999).
Mutagenesis of the phosphatase sequence motif in diacylglycerol pyrophosphate phosphatase from Saccharomyces cerevisiae.
|
| |
Biochemistry,
38,
14606-14613.
|
 |
|
|
|
|
 |
J.Littlechild
(1999).
Haloperoxidases and their role in biotransformation reactions.
|
| |
Curr Opin Chem Biol,
3,
28-34.
|
 |
|
|
|
|
 |
A.A.Brindley,
A.R.Dalby,
M.N.Isupov,
and
J.A.Littlechild
(1998).
Preliminary X-ray analysis of a new crystal form of the vanadium-dependent bromoperoxidase from Corallina officinalis.
|
| |
Acta Crystallogr D Biol Crystallogr,
54,
454-457.
|
 |
|
|
|
|
 |
A.Butler
(1998).
Vanadium haloperoxidases.
|
| |
Curr Opin Chem Biol,
2,
279-285.
|
 |
|
|
|
|
 |
D.W.Leung,
C.K.Tompkins,
and
T.White
(1998).
Molecular cloning of two alternatively spliced forms of human phosphatidic acid phosphatase cDNAs that are differentially expressed in normal and tumor cells.
|
| |
DNA Cell Biol,
17,
377-385.
|
 |
|
|
|
|
 |
M.Gerstein,
and
H.Hegyi
(1998).
Comparing genomes in terms of protein structure: surveys of a finite parts list.
|
| |
FEMS Microbiol Rev,
22,
277-304.
|
 |
|
|
|
|
 |
A.F.Neuwald
(1997).
An unexpected structural relationship between integral membrane phosphatases and soluble haloperoxidases.
|
| |
Protein Sci,
6,
1764-1767.
|
 |
|
|
|
|
 |
A.Messerschmidt,
L.Prade,
and
R.Wever
(1997).
Implications for the catalytic mechanism of the vanadium-containing enzyme chloroperoxidase from the fungus Curvularia inaequalis by X-ray structures of the native and peroxide form.
|
| |
Biol Chem,
378,
309-315.
|
 |
|
PDB codes:
|
 |
|
|
|
|
|
 |
J.Stukey,
and
G.M.Carman
(1997).
Identification of a novel phosphatase sequence motif.
|
| |
Protein Sci,
6,
469-472.
|
 |
|
|
|
|
 |
M.Zhang,
M.Zhou,
R.L.Van Etten,
and
C.V.Stauffacher
(1997).
Crystal structure of bovine low molecular weight phosphotyrosyl phosphatase complexed with the transition state analog vanadate.
|
| |
Biochemistry,
36,
15-23.
|
 |
|
PDB codes:
|
 |
|
|
|
|
|
 |
W.Hemrika,
R.Renirie,
H.L.Dekker,
P.Barnett,
and
R.Wever
(1997).
From phosphatases to vanadium peroxidases: a similar architecture of the active site.
|
| |
Proc Natl Acad Sci U S A,
94,
2145-2149.
|
 |
|
|
|
|
 |
A.Volbeda,
J.C.Fontecilla-Camps,
and
M.Frey
(1996).
Novel metal sites in protein structures.
|
| |
Curr Opin Struct Biol,
6,
804-812.
|
 |
|
 |
 |
|
The most recent references are shown first.
Citation data come partly from CiteXplore and partly
from an automated harvesting procedure. Note that this is likely to be
only a partial list as not all journals are covered by
either method. However, we are continually building up the citation data
so more and more references will be included with time.
Where a reference describes a PDB structure, the PDB
code is
shown on the right.
|
 |

