
|

|

|
|
 |
Contents |
 |
|
|

|

|
|
|
|
|
|
|
|
* Residue conservation analysis
|
|
|
 |
|
|
 |
 |
 |
 |
Enzyme class:
|
 |
Chains A, B:
E.C.6.1.1.20
- phenylalanine--tRNA ligase.
|
|
 |
 |
 |
 |
 |
Reaction:
|
 |
tRNA(Phe) + L-phenylalanine + ATP = L-phenylalanyl-tRNA(Phe) + AMP + diphosphate + H+
|
 |
 |
 |
 |
 |
 tRNA(Phe)
tRNA(Phe)
|
+
|
 L-phenylalanine
L-phenylalanine
|
+
|
 ATP
ATP
|
=
|
 L-phenylalanyl-tRNA(Phe)
L-phenylalanyl-tRNA(Phe)
|
+
|
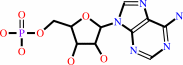 AMP
AMP
|
+
|
 diphosphate
diphosphate
|
+
|
H(+)
|
|
 |
 |
 |
 |
 |
 |
 |
 |
 |
 |
|
Molecule diagrams generated from .mol files obtained from the
KEGG ftp site
|
|
 |
 |
 |
 |
 |
 |
 |
 |
 |
 |
 |
 |
 |
 |
 |

|

|

|
| |
|
|
| |
|
|
Nat Struct Biol
2:537-547
(1995)
|
|
PubMed id:
|
|
|
|

|
| |
|
Structure of phenylalanyl-tRNA synthetase from Thermus thermophilus.
|
|
L.Mosyak,
L.Reshetnikova,
Y.Goldgur,
M.Delarue,
M.G.Safro.
|
|
|

|
| |
ABSTRACT
|
|

|
| |

|
The crystal structure of phenylalanyl-tRNA synthetase from Thermus thermophilus,
solved at 2.9 A resolution, displays (alpha beta)2 subunit organization.
Unexpectedly, both the catalytic alpha- and the non-catalytic beta-subunits
comprise the characteristic fold of the class II active-site domains. The alpha
beta heterodimer contains most of the building blocks so far identified in the
class II synthetases. The presence of an RNA-binding domain, similar to that of
the U1A spliceosomal protein, in the beta-subunit is indicative of structural
relationships among different families of RNA-binding proteins. The structure
suggests a plausible catalytic mechanism which explains why the primary site of
tRNA aminoacylation is different from that of the other class II enzymes.

|

|
|
|

|

|
|
 |
 |
|
 |
 |
 |
 |
 |
 |
 |
 |
 |
|
Literature references that cite this PDB file's key reference
|
|
 |
| |
PubMed id
|
 |
Reference
|
 |
|
|
|
 |
A.Minajigi,
B.Deng,
and
C.S.Francklyn
(2011).
Fidelity escape by the unnatural amino acid β-hydroxynorvaline: an efficient substrate for Escherichia coli threonyl-tRNA synthetase with toxic effects on growth.
|
| |
Biochemistry,
50,
1101-1109.
|
 |
|
|
|
|
 |
I.Mermershtain,
I.Finarov,
L.Klipcan,
N.Kessler,
H.Rozenberg,
and
M.G.Safro
(2011).
Idiosyncrasy and identity in the prokaryotic phe-system: crystal structure of E. coli phenylalanyl-tRNA synthetase complexed with phenylalanine and AMP.
|
| |
Protein Sci,
20,
160-167.
|
 |
|
PDB code:
|
 |
|
|
|
|
|
 |
G.P.Fournier,
and
J.P.Gogarten
(2010).
Rooting the ribosomal tree of life.
|
| |
Mol Biol Evol,
27,
1792-1801.
|
 |
|
|
|
|
 |
I.Finarov,
N.Moor,
N.Kessler,
L.Klipcan,
and
M.G.Safro
(2010).
Structure of human cytosolic phenylalanyl-tRNA synthetase: evidence for kingdom-specific design of the active sites and tRNA binding patterns.
|
| |
Structure,
18,
343-353.
|
 |
|
PDB code:
|
 |
|
|
|
|
|
 |
G.Sharma,
and
E.A.First
(2009).
Thermodynamic Analysis Reveals a Temperature-dependent Change in the Catalytic Mechanism of Bacillus stearothermophilus Tyrosyl-tRNA Synthetase.
|
| |
J Biol Chem,
284,
4179-4190.
|
 |
|
|
|
|
 |
I.Finarov,
N.Moor,
N.Kessler,
and
M.Safro
(2009).
Crystallization and X-ray analysis of human cytoplasmic phenylalanyl-tRNA synthetase.
|
| |
Acta Crystallogr Sect F Struct Biol Cryst Commun,
65,
93-97.
|
 |
|
|
|
|
 |
L.Klipcan,
N.Moor,
N.Kessler,
and
M.G.Safro
(2009).
Eukaryotic cytosolic and mitochondrial phenylalanyl-tRNA synthetases catalyze the charging of tRNA with the meta-tyrosine.
|
| |
Proc Natl Acad Sci U S A,
106,
11045-11048.
|
 |
|
PDB codes:
|
 |
|
|
|
|
|
 |
M.Naganuma,
S.Sekine,
R.Fukunaga,
and
S.Yokoyama
(2009).
Unique protein architecture of alanyl-tRNA synthetase for aminoacylation, editing, and dimerization.
|
| |
Proc Natl Acad Sci U S A,
106,
8489-8494.
|
 |
|
PDB codes:
|
 |
|
|
|
|
|
 |
S.Bilokapic,
N.Ivic,
V.Godinic-Mikulcic,
I.Piantanida,
N.Ban,
and
I.Weygand-Durasevic
(2009).
Idiosyncratic Helix-Turn-Helix Motif in Methanosarcina barkeri Seryl-tRNA Synthetase Has a Critical Architectural Role.
|
| |
J Biol Chem,
284,
10706-10713.
|
 |
|
|
|
|
 |
K.Oki,
K.Sakamoto,
T.Kobayashi,
H.M.Sasaki,
and
S.Yokoyama
(2008).
Transplantation of a tyrosine editing domain into a tyrosyl-tRNA synthetase variant enhances its specificity for a tyrosine analog.
|
| |
Proc Natl Acad Sci U S A,
105,
13298-13303.
|
 |
|
|
|
|
 |
L.Klipcan,
I.Levin,
N.Kessler,
N.Moor,
I.Finarov,
and
M.Safro
(2008).
The tRNA-induced conformational activation of human mitochondrial phenylalanyl-tRNA synthetase.
|
| |
Structure,
16,
1095-1104.
|
 |
|
PDB code:
|
 |
|
|
|
|
|
 |
S.I.Hauenstein,
Y.M.Hou,
and
J.J.Perona
(2008).
The homotetrameric phosphoseryl-tRNA synthetase from Methanosarcina mazei exhibits half-of-the-sites activity.
|
| |
J Biol Chem,
283,
21997-22006.
|
 |
|
|
|
|
 |
Y.G.Gao,
M.Yao,
and
I.Tanaka
(2008).
Structure of protein PH0536 from Pyrococcus horikoshii at 1.7 A resolution reveals a novel assembly of an oligonucleotide/oligosaccharide-binding fold and an alpha-helical bundle.
|
| |
Proteins,
71,
503-508.
|
 |
|
PDB code:
|
 |
|
|
|
|
|
 |
G.Lerman,
and
B.E.Shakhnovich
(2007).
Defining functional distance using manifold embeddings of gene ontology annotations.
|
| |
Proc Natl Acad Sci U S A,
104,
11334-11339.
|
 |
|
|
|
|
 |
I.Levin,
N.Kessler,
N.Moor,
L.Klipcan,
E.Koc,
P.Templeton,
L.Spremulli,
and
M.Safro
(2007).
Purification, crystallization and preliminary X-ray characterization of a human mitochondrial phenylalanyl-tRNA synthetase.
|
| |
Acta Crystallogr Sect F Struct Biol Cryst Commun,
63,
761-764.
|
 |
|
|
|
|
 |
J.Ling,
H.Roy,
and
M.Ibba
(2007).
Mechanism of tRNA-dependent editing in translational quality control.
|
| |
Proc Natl Acad Sci U S A,
104,
72-77.
|
 |
|
|
|
|
 |
R.Fukunaga,
and
S.Yokoyama
(2007).
Structural insights into the first step of RNA-dependent cysteine biosynthesis in archaea.
|
| |
Nat Struct Mol Biol,
14,
272-279.
|
 |
|
PDB codes:
|
 |
|
|
|
|
|
 |
S.Kamtekar,
M.J.Hohn,
H.S.Park,
M.Schnitzbauer,
A.Sauerwald,
D.Söll,
and
T.A.Steitz
(2007).
Toward understanding phosphoseryl-tRNACys formation: the crystal structure of Methanococcus maripaludis phosphoseryl-tRNA synthetase.
|
| |
Proc Natl Acad Sci U S A,
104,
2620-2625.
|
 |
|
PDB code:
|
 |
|
|
|
|
|
 |
H.M.Sasaki,
S.Sekine,
T.Sengoku,
R.Fukunaga,
M.Hattori,
Y.Utsunomiya,
C.Kuroishi,
S.Kuramitsu,
M.Shirouzu,
and
S.Yokoyama
(2006).
Structural and mutational studies of the amino acid-editing domain from archaeal/eukaryal phenylalanyl-tRNA synthetase.
|
| |
Proc Natl Acad Sci U S A,
103,
14744-14749.
|
 |
|
PDB code:
|
 |
|
|
|
|
|
 |
O.Kotik-Kogan,
N.Moor,
D.Tworowski,
and
M.Safro
(2005).
Structural basis for discrimination of L-phenylalanine from L-tyrosine by phenylalanyl-tRNA synthetase.
|
| |
Structure,
13,
1799-1807.
|
 |
|
PDB codes:
|
 |
|
|
|
|
|
 |
P.O'Donoghue,
A.Sethi,
C.R.Woese,
and
Z.A.Luthey-Schulten
(2005).
The evolutionary history of Cys-tRNACys formation.
|
| |
Proc Natl Acad Sci U S A,
102,
19003-19008.
|
 |
|
|
|
|
 |
A.Martins,
and
S.Shuman
(2004).
An RNA ligase from Deinococcus radiodurans.
|
| |
J Biol Chem,
279,
50654-50661.
|
 |
|
|
|
|
 |
D.Beyer,
H.P.Kroll,
R.Endermann,
G.Schiffer,
S.Siegel,
M.Bauser,
J.Pohlmann,
M.Brands,
K.Ziegelbauer,
D.Haebich,
C.Eymann,
and
H.Brötz-Oesterhelt
(2004).
New class of bacterial phenylalanyl-tRNA synthetase inhibitors with high potency and broad-spectrum activity.
|
| |
Antimicrob Agents Chemother,
48,
525-532.
|
 |
|
|
|
|
 |
H.Roy,
J.Ling,
M.Irnov,
and
M.Ibba
(2004).
Post-transfer editing in vitro and in vivo by the beta subunit of phenylalanyl-tRNA synthetase.
|
| |
EMBO J,
23,
4639-4648.
|
 |
|
|
|
|
 |
M.A.Swairjo,
F.J.Otero,
X.L.Yang,
M.A.Lovato,
R.J.Skene,
D.E.McRee,
L.Ribas de Pouplana,
and
P.Schimmel
(2004).
Alanyl-tRNA synthetase crystal structure and design for acceptor-stem recognition.
|
| |
Mol Cell,
13,
829-841.
|
 |
|
PDB code:
|
 |
|
|
|
|
|
 |
A.Seto,
M.Shirouzu,
T.Terada,
K.Murayama,
S.Kuramitsu,
and
S.Yokoyama
(2003).
Crystal structure of a hypothetical protein, TT1725, from Thermus thermophilus HB8 at 1.7 A resolution.
|
| |
Proteins,
53,
768-771.
|
 |
|
PDB code:
|
 |
|
|
|
|
|
 |
S.W.Jordan,
and
J.E.Cronan
(2003).
The Escherichia coli lipB gene encodes lipoyl (octanoyl)-acyl carrier protein:protein transferase.
|
| |
J Bacteriol,
185,
1582-1589.
|
 |
|
|
|
|
 |
A.E.Todd,
C.A.Orengo,
and
J.M.Thornton
(2002).
Sequence and structural differences between enzyme and nonenzyme homologs.
|
| |
Structure,
10,
1435-1451.
|
 |
|
|
|
|
 |
I.A.Vasil'eva,
V.N.Ankilova,
O.I.Lavrik,
and
N.A.Moor
(2002).
tRNA discrimination by T. thermophilus phenylalanyl-tRNA synthetase at the binding step.
|
| |
J Mol Recognit,
15,
188-196.
|
 |
|
|
|
|
 |
L.Renault,
P.Kerjan,
S.Pasqualato,
J.Ménétrey,
J.C.Robinson,
S.Kawaguchi,
D.G.Vassylyev,
S.Yokoyama,
M.Mirande,
and
J.Cherfils
(2001).
Structure of the EMAPII domain of human aminoacyl-tRNA synthetase complex reveals evolutionary dimer mimicry.
|
| |
EMBO J,
20,
570-578.
|
 |
|
PDB codes:
|
 |
|
|
|
|
|
 |
S.Kawaguchi,
J.Müller,
D.Linde,
S.Kuramitsu,
T.Shibata,
Y.Inoue,
D.G.Vassylyev,
and
S.Yokoyama
(2001).
The crystal structure of the ttCsaA protein: an export-related chaperone from Thermus thermophilus.
|
| |
EMBO J,
20,
562-569.
|
 |
|
PDB code:
|
 |
|
|
|
|
|
 |
C.D.Wolfgang,
M.Essand,
J.J.Vincent,
B.Lee,
and
I.Pastan
(2000).
TARP: a nuclear protein expressed in prostate and breast cancer cells derived from an alternate reading frame of the T cell receptor gamma chain locus.
|
| |
Proc Natl Acad Sci U S A,
97,
9437-9442.
|
 |
|
|
|
|
 |
M.Ibba,
and
D.Soll
(2000).
Aminoacyl-tRNA synthesis.
|
| |
Annu Rev Biochem,
69,
617-650.
|
 |
|
|
|
|
 |
S.Onesti,
G.Desogus,
A.Brevet,
J.Chen,
P.Plateau,
S.Blanquet,
and
P.Brick
(2000).
Structural studies of lysyl-tRNA synthetase: conformational changes induced by substrate binding.
|
| |
Biochemistry,
39,
12853-12861.
|
 |
|
PDB codes:
|
 |
|
|
|
|
|
 |
V.Guez,
S.Nair,
A.Chaffotte,
and
H.Bedouelle
(2000).
The anticodon-binding domain of tyrosyl-tRNA synthetase: state of folding and origin of the crystallographic disorder.
|
| |
Biochemistry,
39,
1739-1747.
|
 |
|
|
|
|
 |
M.Stoldt,
J.Wöhnert,
O.Ohlenschläger,
M.Görlach,
and
L.R.Brown
(1999).
The NMR structure of the 5S rRNA E-domain-protein L25 complex shows preformed and induced recognition.
|
| |
EMBO J,
18,
6508-6521.
|
 |
|
PDB code:
|
 |
|
|
|
|
|
 |
G.Simos,
A.Sauer,
F.Fasiolo,
and
E.C.Hurt
(1998).
A conserved domain within Arc1p delivers tRNA to aminoacyl-tRNA synthetases.
|
| |
Mol Cell,
1,
235-242.
|
 |
|
|
|
|
 |
L.Ribas de Pouplana,
D.Buechter,
N.Y.Sardesai,
and
P.Schimmel
(1998).
Functional analysis of peptide motif for RNA microhelix binding suggests new family of RNA-binding domains.
|
| |
EMBO J,
17,
5449-5457.
|
 |
|
|
|
|
 |
M.Stoldt,
J.Wöhnert,
M.Görlach,
and
L.R.Brown
(1998).
The NMR structure of Escherichia coli ribosomal protein L25 shows homology to general stress proteins and glutaminyl-tRNA synthetases.
|
| |
EMBO J,
17,
6377-6384.
|
 |
|
PDB code:
|
 |
|
|
|
|
|
 |
R.Furter
(1998).
Expansion of the genetic code: site-directed p-fluoro-phenylalanine incorporation in Escherichia coli.
|
| |
Protein Sci,
7,
419-426.
|
 |
|
|
|
|
 |
A.Aberg,
A.Yaremchuk,
M.Tukalo,
B.Rasmussen,
and
S.Cusack
(1997).
Crystal structure analysis of the activation of histidine by Thermus thermophilus histidyl-tRNA synthetase.
|
| |
Biochemistry,
36,
3084-3094.
|
 |
|
PDB codes:
|
 |
|
|
|
|
|
 |
A.Lechler,
A.Martin,
T.Zuleeg,
S.Limmer,
and
R.Kreutzer
(1997).
A biologically active 53 kDa fragment of overproduced alanyl-tRNA synthetase from Thermus thermophilus HB8 specifically interacts with tRNA Ala acceptor helix.
|
| |
Nucleic Acids Res,
25,
2737-2744.
|
 |
|
|
|
|
 |
B.F.Clark,
and
J.Nyborg
(1997).
The ternary complex of EF-Tu and its role in protein biosynthesis.
|
| |
Curr Opin Struct Biol,
7,
110-116.
|
 |
|
|
|
|
 |
M.Bycroft,
T.J.Hubbard,
M.Proctor,
S.M.Freund,
and
A.G.Murzin
(1997).
The solution structure of the S1 RNA binding domain: a member of an ancient nucleic acid-binding fold.
|
| |
Cell,
88,
235-242.
|
 |
|
PDB code:
|
 |
|
|
|
|
|
 |
S.Cusack
(1997).
Aminoacyl-tRNA synthetases.
|
| |
Curr Opin Struct Biol,
7,
881-889.
|
 |
|
|
|
|
 |
S.Quevillon,
F.Agou,
J.C.Robinson,
and
M.Mirande
(1997).
The p43 component of the mammalian multi-synthetase complex is likely to be the precursor of the endothelial monocyte-activating polypeptide II cytokine.
|
| |
J Biol Chem,
272,
32573-32579.
|
 |
|
|
|
|
 |
S.Rowsell,
R.A.Pauptit,
A.D.Tucker,
R.G.Melton,
D.M.Blow,
and
P.Brick
(1997).
Crystal structure of carboxypeptidase G2, a bacterial enzyme with applications in cancer therapy.
|
| |
Structure,
5,
337-347.
|
 |
|
PDB code:
|
 |
|
|
|
|
|
 |
S.Sen,
H.Zhou,
T.Ripmaster,
W.N.Hittelman,
P.Schimmel,
and
R.A.White
(1997).
Expression of a gene encoding a tRNA synthetase-like protein is enhanced in tumorigenic human myeloid leukemia cells and is cell cycle stage- and differentiation-dependent.
|
| |
Proc Natl Acad Sci U S A,
94,
6164-6169.
|
 |
|
|
|
|
 |
Y.Goldgur,
L.Mosyak,
L.Reshetnikova,
V.Ankilova,
O.Lavrik,
S.Khodyreva,
and
M.Safro
(1997).
The crystal structure of phenylalanyl-tRNA synthetase from thermus thermophilus complexed with cognate tRNAPhe.
|
| |
Structure,
5,
59-68.
|
 |
|
PDB code:
|
 |
|
|
|
|
|
 |
F.Agou,
J.P.Waller,
and
M.Mirande
(1996).
Expression of rat aspartyl-tRNA synthetase in Saccharomyces cerevisiae. Role of the NH2-terminal polypeptide extension on enzyme activity and stability.
|
| |
J Biol Chem,
271,
29295-29303.
|
 |
|
|
|
|
 |
S.P.Hale,
and
P.Schimmel
(1996).
Protein synthesis editing by a DNA aptamer.
|
| |
Proc Natl Acad Sci U S A,
93,
2755-2758.
|
 |
|
|
|
|
 |
W.Freist,
H.Sternbach,
and
F.Cramer
(1996).
Phenylalanyl-tRNA synthetase from yeast and its discrimination of 19 amino acids in aminoacylation of tRNA(Phe)-C-C-A and tRNA(Phe)-C-C-A(3'NH2).
|
| |
Eur J Biochem,
240,
526-531.
|
 |
|
|
|
|
 |
M.Safro,
and
L.Mosyak
(1995).
Structural similarities in the noncatalytic domains of phenylalanyl-tRNA and biotin synthetases.
|
| |
Protein Sci,
4,
2429-2432.
|
 |
|
|
|
|
 |
R.Kreutzer,
D.Kern,
R.Giegé,
and
J.Rudinger
(1995).
Footprinting of tRNA(Phe) transcripts from Thermus thermophilus HB8 with the homologous phenylalanyl-tRNA synthetase reveals a novel mode of interaction.
|
| |
Nucleic Acids Res,
23,
4598-4602.
|
 |
|
 |
 |
|
The most recent references are shown first.
Citation data come partly from CiteXplore and partly
from an automated harvesting procedure. Note that this is likely to be
only a partial list as not all journals are covered by
either method. However, we are continually building up the citation data
so more and more references will be included with time.
Where a reference describes a PDB structure, the PDB
code is
shown on the right.
|
 |

