 |
PDBsum entry 1peh

|
|
|

|
 |

|

|

|

|

|

|

|

|

|

|
 |

|

|

|
 |
 |
 |
 |
 |
 |
 |
 |
 |
 |
 |
 |
 |
 |
 |
 |

|

|

|

|

|

|

|

|

|
|
Nucleotidyltransferase
|
PDB id
|
|

|

|
1peh
|

|

|

|

|

|

|

|

|

|

|

|

|

|

|

|

|

|

|

|
|
 |
Contents |
 |
|
|

|

|
|
|
|
* Residue conservation analysis
|
|
|
|
 |
|
|
 |
 |
 |
 |
Enzyme class:
|
 |
E.C.2.7.7.15
- choline-phosphate cytidylyltransferase.
|
|
 |
 |
 |
 |
 |
Reaction:
|
 |
phosphocholine + CTP + H+ = CDP-choline + diphosphate
|
 |
 |
 |
 |
 |
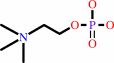 phosphocholine
phosphocholine
|
+
|
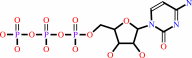 CTP
CTP
|
+
|
H(+)
|
=
|
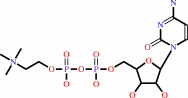 CDP-choline
CDP-choline
|
+
|
 diphosphate
diphosphate
|
|
 |
 |
 |
 |
 |
 |
 |
 |
 |
 |
|
Molecule diagrams generated from .mol files obtained from the
KEGG ftp site
|
|
 |
 |
 |
 |
 |
 |
 |
 |
 |
 |
 |
 |
 |
 |
 |

|

|

|
| |
|
|
| |
|
DOI no:
|
Biochemistry
35:11975-11984
(1996)
|
|
PubMed id:
|
|
|
|

|
| |
|
Structure of the membrane binding domain of CTP:phosphocholine cytidylyltransferase.
|
|
S.J.Dunne,
R.B.Cornell,
J.E.Johnson,
N.R.Glover,
A.S.Tracey.
|
|
|

|
| |
ABSTRACT
|
|

|
| |

|
It has been proposed that the domain of the regulatory enzyme,
CTP:phosphocholine cytidylyltransferase, which mediates reversible binding of
the enzyme to membranes, is an amphipathic alpha-helix of approximately 60 amino
acid residues and that this domain is adjacent to the putative active site
domain of this enzyme. Circular dichroism indicated that the secondary
structures of two overlapping peptides spanning this region were predominantly
alpha-helical in the presence of PG vesicles or sodium dodecyl sulfate micelles.
Interproton distances were obtained from two-dimensional NMR spectroscopic
measurements to solve the structures of these two peptides. The C-terminal 22
amino acid peptide segment (corresponding to Val267-Ser288) was a well-defined
alpha-helix over its length. The N-terminal 33-mer (corresponding to
Asn236-Glu268) was composed of an alpha-helix from Glu243 to Lys266, a
well-structured bend of about 50 degrees at Tyr240-His241-Leu242, and an
N-terminal four-residue helix. It is proposed that the three residues involved
in generating the bend act as the hinge between the catalytic and regulatory
domains. The nonpolar faces of the 33-mer and 22-mer were interrupted by Ser260,
Ser271, and Ser282. These residues may serve to limit the hydrophobicity and
facilitate reversible and lipid-selective membrane binding. The hydrophobic
faces of the helices were flanked by a set of basic amino acid residues on one
side and basic amino acid residues interspersed with glutamates on the other.
The disposition of these side chains gives clues to the basis for the
specificities of these peptides for anionic surfaces.

|

|
|
|

|

|
|
 |
 |
|
 |
 |
 |
 |
 |
 |
 |
 |
 |
|
Literature references that cite this PDB file's key reference
|
|
 |
| |
PubMed id
|
 |
Reference
|
 |
|
|
|
 |
J.D.Braker,
K.J.Hodel,
D.R.Mullins,
and
J.A.Friesen
(2009).
Identification of hydrophobic amino acids required for lipid activation of C. elegans CTP:phosphocholine cytidylyltransferase.
|
| |
Arch Biochem Biophys,
492,
10-16.
|
 |
|
|
|
|
 |
N.Testerink,
M.H.van der Sanden,
M.Houweling,
J.B.Helms,
and
A.B.Vaandrager
(2009).
Depletion of phosphatidylcholine affects endoplasmic reticulum morphology and protein traffic at the Golgi complex.
|
| |
J Lipid Res,
50,
2182-2192.
|
 |
|
|
|
|
 |
R.Inatsugi,
H.Kawai,
Y.Yamaoka,
Y.Yu,
A.Sekiguchi,
M.Nakamura,
and
I.Nishida
(2009).
Isozyme-specific modes of activation of CTP:phosphorylcholine cytidylyltransferase in Arabidopsis thaliana at low temperature.
|
| |
Plant Cell Physiol,
50,
1727-1735.
|
 |
|
|
|
|
 |
K.Gehrig,
R.B.Cornell,
and
N.D.Ridgway
(2008).
Expansion of the Nucleoplasmic Reticulum Requires the Coordinated Activity of Lamins and CTP:Phosphocholine Cytidylyltransferase {alpha}.
|
| |
Mol Biol Cell,
19,
237-247.
|
 |
|
|
|
|
 |
M.Mihajlovic,
and
T.Lazaridis
(2008).
Membrane-bound structure and energetics of alpha-synuclein.
|
| |
Proteins,
70,
761-778.
|
 |
|
|
|
|
 |
G.Drin,
J.F.Casella,
R.Gautier,
T.Boehmer,
T.U.Schwartz,
and
B.Antonny
(2007).
A general amphipathic alpha-helical motif for sensing membrane curvature.
|
| |
Nat Struct Mol Biol,
14,
138-146.
|
 |
|
|
|
|
 |
M.Agassandian,
J.Zhou,
L.A.Tephly,
A.J.Ryan,
A.B.Carter,
and
R.K.Mallampalli
(2005).
Oxysterols inhibit phosphatidylcholine synthesis via ERK docking and phosphorylation of CTP:phosphocholine cytidylyltransferase.
|
| |
J Biol Chem,
280,
21577-21587.
|
 |
|
|
|
|
 |
M.J.Bogan,
G.R.Agnes,
F.Pio,
and
R.B.Cornell
(2005).
Interdomain and membrane interactions of CTP:phosphocholine cytidylyltransferase revealed via limited proteolysis and mass spectrometry.
|
| |
J Biol Chem,
280,
19613-19624.
|
 |
|
|
|
|
 |
M.H.van der Sanden,
H.Meems,
M.Houweling,
J.B.Helms,
and
A.B.Vaandrager
(2004).
Induction of CCAAT/enhancer-binding protein (C/EBP)-homologous protein/growth arrest and DNA damage-inducible protein 153 expression during inhibition of phosphatidylcholine synthesis is mediated via activation of a C/EBP-activating transcription factor-responsive element.
|
| |
J Biol Chem,
279,
52007-52015.
|
 |
|
|
|
|
 |
M.Xie,
J.L.Smith,
Z.Ding,
D.Zhang,
and
R.B.Cornell
(2004).
Membrane binding modulates the quaternary structure of CTP:phosphocholine cytidylyltransferase.
|
| |
J Biol Chem,
279,
28817-28825.
|
 |
|
|
|
|
 |
S.Jackowski,
J.E.Rehg,
Y.M.Zhang,
J.Wang,
K.Miller,
P.Jackson,
and
M.A.Karim
(2004).
Disruption of CCTbeta2 expression leads to gonadal dysfunction.
|
| |
Mol Cell Biol,
24,
4720-4733.
|
 |
|
|
|
|
 |
J.E.Johnson,
M.Xie,
L.M.Singh,
R.Edge,
and
R.B.Cornell
(2003).
Both acidic and basic amino acids in an amphitropic enzyme, CTP:phosphocholine cytidylyltransferase, dictate its selectivity for anionic membranes.
|
| |
J Biol Chem,
278,
514-522.
|
 |
|
|
|
|
 |
S.Taneva,
J.E.Johnson,
and
R.B.Cornell
(2003).
Lipid-induced conformational switch in the membrane binding domain of CTP:phosphocholine cytidylyltransferase: a circular dichroism study.
|
| |
Biochemistry,
42,
11768-11776.
|
 |
|
|
|
|
 |
T.H.Szeto,
S.L.Rowland,
C.L.Habrukowich,
and
G.F.King
(2003).
The MinD membrane targeting sequence is a transplantable lipid-binding helix.
|
| |
J Biol Chem,
278,
40050-40056.
|
 |
|
|
|
|
 |
T.H.Szeto,
S.L.Rowland,
L.I.Rothfield,
and
G.F.King
(2002).
Membrane localization of MinD is mediated by a C-terminal motif that is conserved across eubacteria, archaea, and chloroplasts.
|
| |
Proc Natl Acad Sci U S A,
99,
15693-15698.
|
 |
|
|
|
|
 |
V.Brass,
E.Bieck,
R.Montserret,
B.Wölk,
J.A.Hellings,
H.E.Blum,
F.Penin,
and
D.Moradpour
(2002).
An amino-terminal amphipathic alpha-helix mediates membrane association of the hepatitis C virus nonstructural protein 5A.
|
| |
J Biol Chem,
277,
8130-8139.
|
 |
|
|
|
|
 |
A.Lykidis,
P.Jackson,
and
S.Jackowski
(2001).
Lipid activation of CTP: phosphocholine cytidylyltransferase alpha: characterization and identification of a second activation domain.
|
| |
Biochemistry,
40,
494-503.
|
 |
|
|
|
|
 |
B.C.Tan,
K.Cline,
and
D.R.McCarty
(2001).
Localization and targeting of the VP14 epoxy-carotenoid dioxygenase to chloroplast membranes.
|
| |
Plant J,
27,
373-382.
|
 |
|
|
|
|
 |
G.S.Attard,
R.H.Templer,
W.S.Smith,
A.N.Hunt,
and
S.Jackowski
(2000).
Modulation of CTP:phosphocholine cytidylyltransferase by membrane curvature elastic stress.
|
| |
Proc Natl Acad Sci U S A,
97,
9032-9036.
|
 |
|
|
|
|
 |
G.W.Buchko,
A.Rozek,
P.Kanda,
M.A.Kennedy,
and
R.J.Cushley
(2000).
Structural studies of a baboon (Papio sp.) plasma protein inhibitor of cholesteryl ester transferase.
|
| |
Protein Sci,
9,
1548-1558.
|
 |
|
PDB code:
|
 |
|
|
|
|
|
 |
R.B.Cornell,
and
I.C.Northwood
(2000).
Regulation of CTP:phosphocholine cytidylyltransferase by amphitropism and relocalization.
|
| |
Trends Biochem Sci,
25,
441-447.
|
 |
|
|
|
|
 |
A.Lykidis,
I.Baburina,
and
S.Jackowski
(1999).
Distribution of CTP:phosphocholine cytidylyltransferase (CCT) isoforms. Identification of a new CCTbeta splice variant.
|
| |
J Biol Chem,
274,
26992-27001.
|
 |
|
|
|
|
 |
C.Chen,
K.T.Seow,
K.Guo,
L.P.Yaw,
and
S.C.Lin
(1999).
The membrane association domain of RGS16 contains unique amphipathic features that are conserved in RGS4 and RGS5.
|
| |
J Biol Chem,
274,
19799-19806.
|
 |
|
|
|
|
 |
T.Ahola,
A.Lampio,
P.Auvinen,
and
L.Kääriäinen
(1999).
Semliki Forest virus mRNA capping enzyme requires association with anionic membrane phospholipids for activity.
|
| |
EMBO J,
18,
3164-3172.
|
 |
|
|
|
|
 |
A.Lykidis,
K.G.Murti,
and
S.Jackowski
(1998).
Cloning and characterization of a second human CTP:phosphocholine cytidylyltransferase.
|
| |
J Biol Chem,
273,
14022-14029.
|
 |
|
|
|
|
 |
J.E.Johnson,
N.M.Rao,
S.W.Hui,
and
R.B.Cornell
(1998).
Conformation and lipid binding properties of four peptides derived from the membrane-binding domain of CTP:phosphocholine cytidylyltransferase.
|
| |
Biochemistry,
37,
9509-9519.
|
 |
|
|
|
|
 |
R.S.Roy,
S.Kim,
J.D.Baleja,
and
C.T.Walsh
(1998).
Role of the microcin B17 propeptide in substrate recognition: solution structure and mutational analysis of McbA1-26.
|
| |
Chem Biol,
5,
217-228.
|
 |
|
PDB code:
|
 |
|
|
|
|
|
 |
S.P.Srinivasa,
L.S.Bernstein,
K.J.Blumer,
and
M.E.Linder
(1998).
Plasma membrane localization is required for RGS4 function in Saccharomyces cerevisiae.
|
| |
Proc Natl Acad Sci U S A,
95,
5584-5589.
|
 |
|
|
|
|
 |
A.K.Hinderliter,
A.R.Dibble,
R.L.Biltonen,
and
J.J.Sando
(1997).
Activation of protein kinase C by coexisting diacylglycerol-enriched and diacylglycerol-poor lipid domains.
|
| |
Biochemistry,
36,
6141-6148.
|
 |
|
|
|
|
 |
A.Rozek,
G.W.Buchko,
P.Kanda,
and
R.J.Cushley
(1997).
Conformational studies of the N-terminal lipid-associating domain of human apolipoprotein C-I by CD and 1H NMR spectroscopy.
|
| |
Protein Sci,
6,
1858-1868.
|
 |
|
PDB code:
|
 |
|
|
|
|
|
 |
R.S.Arnold,
A.A.DePaoli-Roach,
and
R.B.Cornell
(1997).
Binding of CTP:phosphocholine cytidylyltransferase to lipid vesicles: diacylglycerol and enzyme dephosphorylation increase the affinity for negatively charged membranes.
|
| |
Biochemistry,
36,
6149-6156.
|
 |
|
 |
 |
|
The most recent references are shown first.
Citation data come partly from CiteXplore and partly
from an automated harvesting procedure. Note that this is likely to be
only a partial list as not all journals are covered by
either method. However, we are continually building up the citation data
so more and more references will be included with time.
Where a reference describes a PDB structure, the PDB
code is
shown on the right.
|
 |

