
|

|

|
|
 |
Contents |
 |
|
|

|

|

|

|

|

|

|

 514 a.a.
514 a.a.
|
 |

|

|

|

|

|

|

|

 227 a.a.
227 a.a.
|
 |

|

|

|

|

|

|

|

 261 a.a.
261 a.a.
|
 |

|

|

|

|

|

|

|

 144 a.a.
144 a.a.
|
 |

|

|

|

|

|

|

|

 109 a.a.
109 a.a.
|
 |

|

|

|

|

|

|

|

 98 a.a.
98 a.a.
|
 |

|

|

|

|

|

|

|

 84 a.a.
84 a.a.
|
 |

|

|

|

|

|

|

|

 75 a.a.
75 a.a.
|
 |

|

|

|

|

|

|

|

 73 a.a.
73 a.a.
|
 |

|

|

|

|

|

|

|

 56 a.a.
56 a.a.
|
 |

|

|

|

|

|

|

|

 49 a.a.
49 a.a.
|
 |

|

|

|

|

|

|

|

 47 a.a.
47 a.a.
|
 |

|

|

|

|

|

|

|

 43 a.a.
43 a.a.
|
 |

|

|
|
|
|

|

|

|

|

|
 _CU
×6 _CU
×6
|
 |

|

|

|

|

|

|

|
 _ZN
×2 _ZN
×2
|
 |

|

|

|

|

|

|

|
 _MG
×2 _MG
×2
|
 |

|

|

|

|

|

|

|
 _NA
×2 _NA
×2
|
 |

|

|
|
|
* Residue conservation analysis
|
|
|

|
|
PDB id:
|
 |
|
 |
| Name: |
 |
Oxidoreductase
|
 |
|
Title:
|
 |
Bovine heart cytochromE C oxidase in carbon monoxide-bound state
|
|
Structure:
|
 |
CytochromE C oxidase. Chain: a, n. Synonym: ferrocytochrome c\:oxygen oxidoreductase. Other_details: this enzyme is a hybrid protein complex and is a homodimer. Carbon monoxide-bound state.. CytochromE C oxidase. Chain: b, o. Synonym: ferrocytochrome c\:oxygen oxidoreductase. Other_details: this enzyme is a hybrid protein complex and is a
|
|
Source:
|
 |
Bos taurus. Cattle. Organism_taxid: 9913. Organ: heart. Tissue: heart muscle. Organelle: mitochondrion. Organelle: mitochondrion
|
|
Biol. unit:
|
 |
26mer (from
)
|
|
Resolution:
|
 |
|
2.80Å
|
R-factor:
|
0.213
|
R-free:
|
0.256
|
|
|
Authors:
|
 |
T.Tsukihara,M.Yao
|
Key ref:

|
 |
S.Yoshikawa
et al.
(1998).
Redox-coupled crystal structural changes in bovine heart cytochrome c oxidase.
Science,
280,
1723-1729.
PubMed id:
DOI:

|
 |
|
Date:
|
 |
|
09-Jul-98
|
Release date:
|
22-Jul-99
|
|
|

|

|
|
PROCHECK
|

|

|
|
|
Headers
|
 |
|
|
References
|
|
|
|

|

|
|
P00396
(COX1_BOVIN) -
Cytochrome c oxidase subunit 1 from Bos taurus
|
|

|

|
Seq:
Struc:
|
 |
 |
 |
514 a.a.
514 a.a.
|

|

|

|

|
|

|

|

|
 |
 |

|

|

|
|
P68530
(COX2_BOVIN) -
Cytochrome c oxidase subunit 2 from Bos taurus
|
|

|

|
Seq:
Struc:
|
 |
 |
 |
227 a.a.
227 a.a.
|

|

|

|

|
|

|

|

|
 |
 |

|

|

|
|
P00415
(COX3_BOVIN) -
Cytochrome c oxidase subunit 3 from Bos taurus
|
|

|

|
Seq:
Struc:
|
 |
 |
 |
261 a.a.
261 a.a.*
|

|

|

|

|
|

|

|

|
 |
 |

|

|

|
|
P00423
(COX41_BOVIN) -
Cytochrome c oxidase subunit 4 isoform 1, mitochondrial from Bos taurus
|
|

|

|
Seq:
Struc:
|
 |
 |
 |
169 a.a.
144 a.a.
|

|

|

|

|
|

|

|

|
 |
 |

|

|

|
|
P00426
(COX5A_BOVIN) -
Cytochrome c oxidase subunit 5A, mitochondrial from Bos taurus
|
|

|

|
Seq:
Struc:
|
 |
 |
 |
152 a.a.
109 a.a.
|

|

|

|

|
|

|

|

|
 |
 |

|

|

|
|
P00428
(COX5B_BOVIN) -
Cytochrome c oxidase subunit 5B, mitochondrial from Bos taurus
|
|

|

|
Seq:
Struc:
|
 |
 |
 |
129 a.a.
98 a.a.
|

|

|

|

|
|

|

|

|
 |
 |

|

|

|
|
P07471
(CX6A2_BOVIN) -
Cytochrome c oxidase subunit 6A2, mitochondrial from Bos taurus
|
|

|

|
Seq:
Struc:
|
 |
 |
 |
97 a.a.
84 a.a.
|

|

|

|

|
|

|

|

|
 |
 |

|

|

|
|
P00429
(CX6B1_BOVIN) -
Cytochrome c oxidase subunit 6B1 from Bos taurus
|
|

|

|
Seq:
Struc:
|
 |
 |
 |
86 a.a.
75 a.a.
|

|

|

|

|
|

|

|

|
 |
 |

|

|

|
|
P04038
(COX6C_BOVIN) -
Cytochrome c oxidase subunit 6C from Bos taurus
|
|

|

|
Seq:
Struc:
|
 |
 |
 |
74 a.a.
73 a.a.
|

|

|

|

|
|

|

|

|
 |
 |

|

|

|
|
P07470
(CX7A1_BOVIN) -
Cytochrome c oxidase subunit 7A1, mitochondrial from Bos taurus
|
|

|

|
Seq:
Struc:
|
 |
 |
 |
80 a.a.
56 a.a.
|

|

|

|

|
|

|

|

|
 |
 |

|

|

|
|
P13183
(COX7B_BOVIN) -
Cytochrome c oxidase subunit 7B, mitochondrial from Bos taurus
|
|

|

|
Seq:
Struc:
|
 |
 |
 |
80 a.a.
49 a.a.
|

|

|

|

|
|

|

|

|
 |
 |

|
 |
|
|
 |
 |
 |
 |
Enzyme class 2:
|
 |
Chains A, B, C, N, O, P:
E.C.7.1.1.9
- cytochrome-c oxidase.
|
|
 |
 |
 |
 |
 |
Reaction:
|
 |
4 Fe(II)-[cytochrome c] + O2 + 8 H+(in) = 4 Fe(III)-[cytochrome c] + 2 H2O + 4 H+(out)
|
 |
 |
 |
 |
 |
4
×
Fe(II)-[cytochrome c]
Bound ligand (Het Group name = )
matches with 50.60% similarity
|
+
|
 O2
O2
|
+
|
8
×
H(+)(in)
|
=
|
4
×
Fe(III)-[cytochrome c]
|
+
|
2
×
H2O
|
+
|
4
×
H(+)(out)
|
|
 |
 |
 |
 |
 |
 |
 |
 |
Cofactor:
|
 |
Cu cation
|
 |
 |
 |
 |
 |
Enzyme class 3:
|
 |
Chains D, E, F, G, H, I, J, K, L, M, Q, R, S, T, U, V, W, X, Y, Z:
E.C.1.9.3.1
- Transferred entry: 7.1.1.9.
|
|
 |
 |
 |
 |
 |
Reaction:
|
 |
4 ferrocytochrome c + O2 + 4 H+ = 4 ferricytochrome c + 2 H2O
|
 |
 |
 |
 |
 |
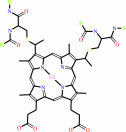 4
×
ferrocytochrome c
4
×
ferrocytochrome c
|
+
|
 O(2)
O(2)
|
+
|
4
×
H(+)
|
=
|
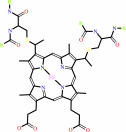 4
×
ferricytochrome c
4
×
ferricytochrome c
|
+
|
2
×
H(2)O
|
|
 |
 |
 |
 |
 |
 |
 |
 |
Cofactor:
|
 |
Cu cation
|
 |
 |
 |
 |
 |
 |
 |
|
Note, where more than one E.C. class is given (as above), each may
correspond to a different protein domain or, in the case of polyprotein
precursors, to a different mature protein.
|
|
 |
|
Molecule diagrams generated from .mol files obtained from the
KEGG ftp site
|
|
 |
 |
 |
 |
 |
 |
 |
 |
 |
 |
 |
 |
 |
 |
 |

|

|

|
| |
|
|
| |
|
DOI no:
|
Science
280:1723-1729
(1998)
|
|
PubMed id:
|
|
|
|

|
| |
|
Redox-coupled crystal structural changes in bovine heart cytochrome c oxidase.
|
|
S.Yoshikawa,
K.Shinzawa-Itoh,
R.Nakashima,
R.Yaono,
E.Yamashita,
N.Inoue,
M.Yao,
M.J.Fei,
C.P.Libeu,
T.Mizushima,
H.Yamaguchi,
T.Tomizaki,
T.Tsukihara.
|
|
|

|
| |
ABSTRACT
|
|

|
| |

|
Crystal structures of bovine heart cytochrome c oxidase in the fully oxidized,
fully reduced, azide-bound, and carbon monoxide-bound states were determined at
2.30, 2.35, 2.9, and 2.8 angstrom resolution, respectively. An aspartate residue
apart from the O2 reduction site exchanges its effective accessibility to the
matrix aqueous phase for one to the cytosolic phase concomitantly with a
significant decrease in the pK of its carboxyl group, on reduction of the metal
sites. The movement indicates the aspartate as the proton pumping site. A
tyrosine acidified by a covalently linked imidazole nitrogen is a possible
proton donor for the O2 reduction by the enzyme.

|

|
|
|

|
| |
Selected figure(s)
|
|

|
| |
 |
 |

|
 |

|
 |
Figure 2.
Fig. 2. Crystal structures of the Fe[a3]-Cu[B] site in the
fully reduced CO-bound and fully oxidized azide-bound states and
those of^ the Na^+/Ca^2+ and Mg^2+ sites. (F[o]  F[c])
difference Fourier maps for CO bound at Fe[a3]^ at 3 F[c])
difference Fourier maps for CO bound at Fe[a3]^ at 3  level (A)
and azide bridging between Fe[a3] and Cu[B]^ at 2.2 level (A)
and azide bridging between Fe[a3] and Cu[B]^ at 2.2  level (B)
are given where the bound ligands and^ fixed waters are not
included in the F[c] calculation. The difference^ electron
density maps at 4 level (B)
are given where the bound ligands and^ fixed waters are not
included in the F[c] calculation. The difference^ electron
density maps at 4  level (1 level (1
 =
0.0436e^-/Å^3) for the Na^+/Ca^2+ site (C) and for the
Mg^2+ site (D). Violet, purple, and blue balls are the
positions^ of Na^+, Mg^2+, and water, respectively. =
0.0436e^-/Å^3) for the Na^+/Ca^2+ site (C) and for the
Mg^2+ site (D). Violet, purple, and blue balls are the
positions^ of Na^+, Mg^2+, and water, respectively.
|
 |
Figure 3.
Fig. 3. Redox-coupled conformational change in the segment
from Gly^49 to Asn^55. The conformation of the segment in the
fully oxidized form is^ stereoscopically shown in red, and that
in the fully reduced form^ in green. The yellow structure
denotes subunit II with no redox-coupled^ conformational change.
|
 |
|
|

|
| |
The above figures are
reprinted
by permission from the AAAs:
Science
(1998,
280,
1723-1729)
copyright 1998.
|
|
| |
Figures were
selected
by an automated process.
|
|

|

|
|
 |
 |
|
 |
 |
 |
 |
 |
 |
 |
 |
 |
|
Literature references that cite this PDB file's key reference
|
|
 |
| |
PubMed id
|
 |
Reference
|
 |
|
|
|
 |
A.V.Dyuba,
A.M.Arutyunyan,
T.V.Vygodina,
N.V.Azarkina,
A.V.Kalinovich,
Y.A.Sharonov,
and
A.A.Konstantinov
(2011).
Circular dichroism spectra of cytochrome c oxidase.
|
| |
Metallomics,
3,
417-432.
|
 |
|
|
|
|
 |
I.von der Hocht,
J.H.van Wonderen,
F.Hilbers,
H.Angerer,
F.Macmillan,
and
H.Michel
(2011).
Interconversions of P and F intermediates of cytochrome c oxidase from Paracoccus denitrificans.
|
| |
Proc Natl Acad Sci U S A,
108,
3964-3969.
|
 |
|
|
|
|
 |
J.Liu,
L.Qin,
and
S.Ferguson-Miller
(2011).
Crystallographic and online spectral evidence for role of conformational change and conserved water in cytochrome oxidase proton pump.
|
| |
Proc Natl Acad Sci U S A,
108,
1284-1289.
|
 |
|
PDB codes:
|
 |
|
|
|
|
|
 |
S.Yoshikawa,
K.Muramoto,
and
K.Shinzawa-Itoh
(2011).
Proton-pumping mechanism of cytochrome C oxidase.
|
| |
Annu Rev Biophys,
40,
205-223.
|
 |
|
|
|
|
 |
V.L.Davidson
(2011).
Generation of protein-derived redox cofactors by posttranslational modification.
|
| |
Mol Biosyst,
7,
29-37.
|
 |
|
|
|
|
 |
K.McLuskey,
A.W.Roszak,
Y.Zhu,
and
N.W.Isaacs
(2010).
Crystal structures of all-alpha type membrane proteins.
|
| |
Eur Biophys J,
39,
723-755.
|
 |
|
|
|
|
 |
K.Muramoto,
K.Ohta,
K.Shinzawa-Itoh,
K.Kanda,
M.Taniguchi,
H.Nabekura,
E.Yamashita,
T.Tsukihara,
and
S.Yoshikawa
(2010).
Bovine cytochrome c oxidase structures enable O2 reduction with minimization of reactive oxygens and provide a proton-pumping gate.
|
| |
Proc Natl Acad Sci U S A,
107,
7740-7745.
|
 |
|
PDB codes:
|
 |
|
|
|
|
|
 |
K.R.Vinothkumar,
and
R.Henderson
(2010).
Structures of membrane proteins.
|
| |
Q Rev Biophys,
43,
65.
|
 |
|
|
|
|
 |
L.J.Smith,
A.Kahraman,
and
J.M.Thornton
(2010).
Heme proteins--diversity in structural characteristics, function, and folding.
|
| |
Proteins,
78,
2349-2368.
|
 |
|
|
|
|
 |
P.R.Rich,
and
A.Maréchal
(2010).
The mitochondrial respiratory chain.
|
| |
Essays Biochem,
47,
1.
|
 |
|
|
|
|
 |
V.S.Oganesyan,
G.F.White,
S.Field,
S.Marritt,
R.B.Gennis,
L.L.Yap,
and
A.J.Thomson
(2010).
Nitroxide spin labels as EPR reporters of the relaxation and magnetic properties of the heme-copper site in cytochrome bo3, E. coli.
|
| |
J Biol Inorg Chem,
15,
1255-1264.
|
 |
|
|
|
|
 |
Y.Yoshioka,
and
M.Mitani
(2010).
B3LYP study on reduction mechanisms from O2 to H2O at the catalytic sites of fully reduced and mixed-valence bovine cytochrome c oxidases.
|
| |
Bioinorg Chem Appl,
(),
182804.
|
 |
|
|
|
|
 |
Z.Halime,
M.T.Kieber-Emmons,
M.F.Qayyum,
B.Mondal,
T.Gandhi,
S.C.Puiu,
E.E.Chufán,
A.A.Sarjeant,
K.O.Hodgson,
B.Hedman,
E.I.Solomon,
and
K.D.Karlin
(2010).
Heme-copper-dioxygen complexes: toward understanding ligand-environmental effects on the coordination geometry, electronic structure, and reactivity.
|
| |
Inorg Chem,
49,
3629-3645.
|
 |
|
|
|
|
 |
A.Barrientos,
K.Gouget,
D.Horn,
I.C.Soto,
and
F.Fontanesi
(2009).
Suppression mechanisms of COX assembly defects in yeast and human: insights into the COX assembly process.
|
| |
Biochim Biophys Acta,
1793,
97.
|
 |
|
|
|
|
 |
A.Offenbacher,
K.N.White,
I.Sen,
A.G.Oliver,
J.P.Konopelski,
B.A.Barry,
and
O.Einarsdóttir
(2009).
A spectroscopic investigation of a tridentate Cu-complex mimicking the tyrosine-histidine cross-link of cytochrome C oxidase.
|
| |
J Phys Chem B,
113,
7407-7417.
|
 |
|
|
|
|
 |
A.Y.Smirnov,
L.G.Mourokh,
and
F.Nori
(2009).
Kinetics of proton pumping in cytochrome c oxidase.
|
| |
J Chem Phys,
130,
235105.
|
 |
|
|
|
|
 |
B.Kadenbach,
R.Ramzan,
and
S.Vogt
(2009).
Degenerative diseases, oxidative stress and cytochrome c oxidase function.
|
| |
Trends Mol Med,
15,
139-147.
|
 |
|
|
|
|
 |
H.Aoyama,
K.Muramoto,
K.Shinzawa-Itoh,
K.Hirata,
E.Yamashita,
T.Tsukihara,
T.Ogura,
and
S.Yoshikawa
(2009).
A peroxide bridge between Fe and Cu ions in the O2 reduction site of fully oxidized cytochrome c oxidase could suppress the proton pump.
|
| |
Proc Natl Acad Sci U S A,
106,
2165-2169.
|
 |
|
PDB codes:
|
 |
|
|
|
|
|
 |
J.P.Collman,
A.Dey,
C.J.Barile,
S.Ghosh,
and
R.A.Decréau
(2009).
Inhibition of electrocatalytic O(2) reduction of functional CcO models by competitive, non-competitive, and mixed inhibitors.
|
| |
Inorg Chem,
48,
10528-10534.
|
 |
|
|
|
|
 |
J.P.Collman,
A.Dey,
Y.Yang,
S.Ghosh,
and
R.A.Decréau
(2009).
O2 reduction by a functional heme/nonheme bis-iron NOR model complex.
|
| |
Proc Natl Acad Sci U S A,
106,
10528-10533.
|
 |
|
|
|
|
 |
J.P.Collman,
S.Ghosh,
A.Dey,
and
R.A.Decréau
(2009).
Using a functional enzyme model to understand the chemistry behind hydrogen sulfide induced hibernation.
|
| |
Proc Natl Acad Sci U S A,
106,
22090-22095.
|
 |
|
|
|
|
 |
K.Kobayashi,
S.Tagawa,
and
T.Mogi
(2009).
Intramolecular electron transfer processes in Cu(B)-deficient cytochrome bo studied by pulse radiolysis.
|
| |
J Biochem,
145,
685-691.
|
 |
|
|
|
|
 |
K.Kobayashi,
S.Tagawa,
and
T.Mogi
(2009).
Electron transfer processes in subunit I mutants of cytochrome bo quinol oxidase in Escherichia coli.
|
| |
Biosci Biotechnol Biochem,
73,
1599-1603.
|
 |
|
|
|
|
 |
L.Geren,
B.Durham,
and
F.Millett
(2009).
Chapter 28 Use of ruthenium photoreduction techniques to study electron transfer in cytochrome oxidase.
|
| |
Methods Enzymol,
456,
507-520.
|
 |
|
|
|
|
 |
M.E.Mahoney,
A.Oliver,
O.Einarsdóttir,
and
J.P.Konopelski
(2009).
Synthesis of a cyclic pentapeptide mimic of the active site His-Tyr cofactor of cytochrome c oxidase.
|
| |
J Org Chem,
74,
8212-8218.
|
 |
|
|
|
|
 |
P.E.Siegbahn,
and
M.R.Blomberg
(2009).
A combined picture from theory and experiments on water oxidation, oxygen reduction and proton pumping.
|
| |
Dalton Trans,
(),
5832-5840.
|
 |
|
|
|
|
 |
T.Egawa,
H.J.Lee,
R.B.Gennis,
S.R.Yeh,
and
D.L.Rousseau
(2009).
Critical structural role of R481 in cytochrome c oxidase from Rhodobacter sphaeroides.
|
| |
Biochim Biophys Acta,
1787,
1272-1275.
|
 |
|
|
|
|
 |
T.Hayashi,
M.T.Lin,
K.Ganesan,
Y.Chen,
J.A.Fee,
R.B.Gennis,
and
P.Moënne-Loccoz
(2009).
Accommodation of two diatomic molecules in cytochrome bo: insights into NO reductase activity in terminal oxidases.
|
| |
Biochemistry,
48,
883-890.
|
 |
|
|
|
|
 |
T.Mogi
(2009).
Over-expression and characterization of Bacillus subtilis heme O synthase.
|
| |
J Biochem,
145,
669-675.
|
 |
|
|
|
|
 |
A.V.Pisliakov,
P.K.Sharma,
Z.T.Chu,
M.Haranczyk,
and
A.Warshel
(2008).
Electrostatic basis for the unidirectionality of the primary proton transfer in cytochrome c oxidase.
|
| |
Proc Natl Acad Sci U S A,
105,
7726-7731.
|
 |
|
|
|
|
 |
D.G.Isom,
B.R.Cannon,
C.A.Castañeda,
A.Robinson,
and
B.García-Moreno
(2008).
High tolerance for ionizable residues in the hydrophobic interior of proteins.
|
| |
Proc Natl Acad Sci U S A,
105,
17784-17788.
|
 |
|
|
|
|
 |
D.Horn,
and
A.Barrientos
(2008).
Mitochondrial copper metabolism and delivery to cytochrome c oxidase.
|
| |
IUBMB Life,
60,
421-429.
|
 |
|
|
|
|
 |
E.A.Berry,
and
F.A.Walker
(2008).
Bis-histidine-coordinated hemes in four-helix bundles: how the geometry of the bundle controls the axial imidazole plane orientations in transmembrane cytochromes of mitochondrial complexes II and III and related proteins.
|
| |
J Biol Inorg Chem,
13,
481-498.
|
 |
|
|
|
|
 |
E.A.Gorbikova,
I.Belevich,
M.Wikström,
and
M.I.Verkhovsky
(2008).
The proton donor for O-O bond scission by cytochrome c oxidase.
|
| |
Proc Natl Acad Sci U S A,
105,
10733-10737.
|
 |
|
|
|
|
 |
F.M.Ho
(2008).
Uncovering channels in photosystem II by computer modelling: current progress, future prospects, and lessons from analogous systems.
|
| |
Photosynth Res,
98,
503-522.
|
 |
|
|
|
|
 |
I.Belevich,
and
M.I.Verkhovsky
(2008).
Molecular mechanism of proton translocation by cytochrome C oxidase.
|
| |
Antioxid Redox Signal,
10,
1.
|
 |
|
|
|
|
 |
J.A.Fee,
D.A.Case,
and
L.Noodleman
(2008).
Toward a chemical mechanism of proton pumping by the B-type cytochrome c oxidases: application of density functional theory to cytochrome ba3 of Thermus thermophilus.
|
| |
J Am Chem Soc,
130,
15002-15021.
|
 |
|
|
|
|
 |
J.P.Collman,
A.Dey,
R.A.Decreau,
Y.Yang,
A.Hosseini,
E.I.Solomon,
and
T.A.Eberspacher
(2008).
Interaction of nitric oxide with a functional model of cytochrome c oxidase.
|
| |
Proc Natl Acad Sci U S A,
105,
9892-9896.
|
 |
|
|
|
|
 |
J.P.Collman,
and
R.A.Decréau
(2008).
Functional biomimetic models for the active site in the respiratory enzyme cytochrome c oxidase.
|
| |
Chem Commun (Camb),
(),
5065-5076.
|
 |
|
|
|
|
 |
J.Xu,
and
G.A.Voth
(2008).
Redox-coupled proton pumping in cytochrome c oxidase: further insights from computer simulation.
|
| |
Biochim Biophys Acta,
1777,
196-201.
|
 |
|
|
|
|
 |
M.A.Sharpe,
and
S.Ferguson-Miller
(2008).
A chemically explicit model for the mechanism of proton pumping in heme-copper oxidases.
|
| |
J Bioenerg Biomembr,
40,
541-549.
|
 |
|
|
|
|
 |
M.S.Muntyan,
and
D.A.Bloch
(2008).
Study of redox potential in cytochrome c covalently bound to terminal oxidase of alkaliphilic Bacillus pseudofirmus FTU.
|
| |
Biochemistry (Mosc),
73,
107-111.
|
 |
|
|
|
|
 |
M.S.Rogers,
R.Hurtado-Guerrero,
S.J.Firbank,
M.A.Halcrow,
D.M.Dooley,
S.E.Phillips,
P.F.Knowles,
and
M.J.McPherson
(2008).
Cross-link formation of the cysteine 228-tyrosine 272 catalytic cofactor of galactose oxidase does not require dioxygen.
|
| |
Biochemistry,
47,
10428-10439.
|
 |
|
PDB codes:
|
 |
|
|
|
|
|
 |
N.Yeung,
and
Y.Lu
(2008).
One heme, diverse functions: using biosynthetic myoglobin models to gain insights into heme-copper oxidases and nitric oxide reductases.
|
| |
Chem Biodivers,
5,
1437-1448.
|
 |
|
|
|
|
 |
S.E.Hart,
C.J.Howe,
K.Mizuguchi,
and
J.Fernandez-Recio
(2008).
Docking of cytochrome c6 and plastocyanin to the aa3-type cytochrome c oxidase in the cyanobacterium Phormidium laminosum.
|
| |
Protein Eng Des Sel,
21,
689-698.
|
 |
|
|
|
|
 |
V.B.Borisov
(2008).
Interaction of bd-type quinol oxidase from Escherichia coli and carbon monoxide: heme d binds CO with high affinity.
|
| |
Biochemistry (Mosc),
73,
14-22.
|
 |
|
|
|
|
 |
C.Dallacosta,
W.A.Alves,
A.M.da Costa Ferreira,
E.Monzani,
and
L.Casella
(2007).
A new dinuclear heme-copper complex derived from functionalized protoporphyrin IX.
|
| |
Dalton Trans,
(),
2197-2206.
|
 |
|
|
|
|
 |
J.Treuffet,
K.J.Kubarych,
J.C.Lambry,
E.Pilet,
J.B.Masson,
J.L.Martin,
M.H.Vos,
M.Joffre,
and
A.Alexandrou
(2007).
Direct observation of ligand transfer and bond formation in cytochrome c oxidase by using mid-infrared chirped-pulse upconversion.
|
| |
Proc Natl Acad Sci U S A,
104,
15705-15710.
|
 |
|
|
|
|
 |
K.Muramoto,
K.Hirata,
K.Shinzawa-Itoh,
S.Yoko-o,
E.Yamashita,
H.Aoyama,
T.Tsukihara,
and
S.Yoshikawa
(2007).
A histidine residue acting as a controlling site for dioxygen reduction and proton pumping by cytochrome c oxidase.
|
| |
Proc Natl Acad Sci U S A,
104,
7881-7886.
|
 |
|
PDB codes:
|
 |
|
|
|
|
|
 |
K.N.White,
I.Sen,
I.Szundi,
Y.R.Landaverry,
L.E.Bria,
J.P.Konopelski,
M.M.Olmstead,
and
O.Einarsdóttir
(2007).
Synthesis and structural characterization of cross-linked histidine-phenol Cu(ii) complexes as cytochrome c oxidase active site models.
|
| |
Chem Commun (Camb),
(),
3252-3254.
|
 |
|
|
|
|
 |
K.Shimokata,
Y.Katayama,
H.Murayama,
M.Suematsu,
T.Tsukihara,
K.Muramoto,
H.Aoyama,
S.Yoshikawa,
and
H.Shimada
(2007).
The proton pumping pathway of bovine heart cytochrome c oxidase.
|
| |
Proc Natl Acad Sci U S A,
104,
4200-4205.
|
 |
|
|
|
|
 |
M.H.Olsson,
P.E.Siegbahn,
M.R.Blomberg,
and
A.Warshel
(2007).
Exploring pathways and barriers for coupled ET/PT in cytochrome c oxidase: a general framework for examining energetics and mechanistic alternatives.
|
| |
Biochim Biophys Acta,
1767,
244-260.
|
 |
|
|
|
|
 |
P.Nicholls
(2007).
The oxygenase-peroxidase theory of Bach and Chodat and its modern equivalents: change and permanence in scientific thinking as shown by our understanding of the roles of water, peroxide, and oxygen in the functioning of redox enzymes.
|
| |
Biochemistry (Mosc),
72,
1039-1046.
|
 |
|
|
|
|
 |
P.R.Rich,
and
M.Iwaki
(2007).
A comparison of catalytic site intermediates of cytochrome c oxidase and peroxidases.
|
| |
Biochemistry (Mosc),
72,
1047-1055.
|
 |
|
|
|
|
 |
P.Syntichaki,
K.Troulinaki,
and
N.Tavernarakis
(2007).
eIF4E function in somatic cells modulates ageing in Caenorhabditis elegans.
|
| |
Nature,
445,
922-926.
|
 |
|
|
|
|
 |
R.A.Decréau,
J.P.Collman,
Y.Yang,
Y.Yan,
and
N.K.Devaraj
(2007).
Syntheses of hemoprotein models that can be covalently attached onto electrode surfaces by click chemistry.
|
| |
J Org Chem,
72,
2794-2802.
|
 |
|
|
|
|
 |
T.Hayashi,
I.J.Lin,
Y.Chen,
J.A.Fee,
and
P.Moënne-Loccoz
(2007).
Fourier transform infrared characterization of a CuB-nitrosyl complex in cytochrome ba3 from Thermus thermophilus: relevance to NO reductase activity in heme-copper terminal oxidases.
|
| |
J Am Chem Soc,
129,
14952-14958.
|
 |
|
|
|
|
 |
X.Liang,
D.J.Campopiano,
and
P.J.Sadler
(2007).
Metals in membranes.
|
| |
Chem Soc Rev,
36,
968-992.
|
 |
|
|
|
|
 |
Z.O.Wang,
and
D.D.Pollock
(2007).
Coevolutionary patterns in cytochrome c oxidase subunit I depend on structural and functional context.
|
| |
J Mol Evol,
65,
485-495.
|
 |
|
|
|
|
 |
A.C.Dalziel,
C.D.Moyes,
E.Fredriksson,
and
S.C.Lougheed
(2006).
Molecular evolution of cytochrome c oxidase in high-performance fish (teleostei: Scombroidei).
|
| |
J Mol Evol,
62,
319-331.
|
 |
|
|
|
|
 |
D.M.Popovic,
and
A.A.Stuchebrukhov
(2006).
Two conformational states of Glu242 and pKas in bovine cytochrome c oxidase.
|
| |
Photochem Photobiol Sci,
5,
611-620.
|
 |
|
|
|
|
 |
H.Li,
J.Igarashi,
J.Jamal,
W.Yang,
and
T.L.Poulos
(2006).
Structural studies of constitutive nitric oxide synthases with diatomic ligands bound.
|
| |
J Biol Inorg Chem,
11,
753-768.
|
 |
|
PDB codes:
|
 |
|
|
|
|
|
 |
I.Belevich,
M.I.Verkhovsky,
and
M.Wikström
(2006).
Proton-coupled electron transfer drives the proton pump of cytochrome c oxidase.
|
| |
Nature,
440,
829-832.
|
 |
|
|
|
|
 |
I.Bento,
M.A.Carrondo,
and
P.F.Lindley
(2006).
Reduction of dioxygen by enzymes containing copper.
|
| |
J Biol Inorg Chem,
11,
539-547.
|
 |
|
|
|
|
 |
J.S.Winterle,
and
O.Einarsdóttir
(2006).
Photoreactions of cytochrome C oxidase.
|
| |
Photochem Photobiol,
82,
711-719.
|
 |
|
|
|
|
 |
L.Qin,
C.Hiser,
A.Mulichak,
R.M.Garavito,
and
S.Ferguson-Miller
(2006).
Identification of conserved lipid/detergent-binding sites in a high-resolution structure of the membrane protein cytochrome c oxidase.
|
| |
Proc Natl Acad Sci U S A,
103,
16117-16122.
|
 |
|
PDB code:
|
 |
|
|
|
|
|
 |
L.S.Busenlehner,
L.Salomonsson,
P.Brzezinski,
and
R.N.Armstrong
(2006).
Mapping protein dynamics in catalytic intermediates of the redox-driven proton pump cytochrome c oxidase.
|
| |
Proc Natl Acad Sci U S A,
103,
15398-15403.
|
 |
|
|
|
|
 |
M.H.Olsson,
and
A.Warshel
(2006).
Monte Carlo simulations of proton pumps: on the working principles of the biological valve that controls proton pumping in cytochrome c oxidase.
|
| |
Proc Natl Acad Sci U S A,
103,
6500-6505.
|
 |
|
|
|
|
 |
M.R.Blomberg,
and
P.E.Siegbahn
(2006).
Quantum chemistry applied to the mechanisms of transition metal containing enzymes -- cytochrome c oxidase, a particularly challenging case.
|
| |
J Comput Chem,
27,
1373-1384.
|
 |
|
|
|
|
 |
M.Seigneuret
(2006).
Complete predicted three-dimensional structure of the facilitator transmembrane protein and hepatitis C virus receptor CD81: conserved and variable structural domains in the tetraspanin superfamily.
|
| |
Biophys J,
90,
212-227.
|
 |
|
PDB code:
|
 |
|
|
|
|
|
 |
M.W.Bowler,
M.G.Montgomery,
A.G.Leslie,
and
J.E.Walker
(2006).
How azide inhibits ATP hydrolysis by the F-ATPases.
|
| |
Proc Natl Acad Sci U S A,
103,
8646-8649.
|
 |
|
PDB codes:
|
 |
|
|
|
|
|
 |
O.Farver,
E.Grell,
B.Ludwig,
H.Michel,
and
I.Pecht
(2006).
Rates and Equilibrium of CuA to heme a electron transfer in Paracoccus denitrificans cytochrome c oxidase.
|
| |
Biophys J,
90,
2131-2137.
|
 |
|
|
|
|
 |
R.P.Pesavento,
D.A.Pratt,
J.Jeffers,
and
W.A.van der Donk
(2006).
Model studies of the Cu(B) site of cytochrome c oxidase utilizing a Zn(II) complex containing an imidazole-phenol cross-linked ligand.
|
| |
Dalton Trans,
(),
3326-3337.
|
 |
|
|
|
|
 |
R.Schwartz,
and
J.King
(2006).
Frequencies of hydrophobic and hydrophilic runs and alternations in proteins of known structure.
|
| |
Protein Sci,
15,
102-112.
|
 |
|
|
|
|
 |
S.J.Lippard
(2006).
The inorganic side of chemical biology.
|
| |
Nat Chem Biol,
2,
504-507.
|
 |
|
|
|
|
 |
A.E.Bednarski,
S.C.Elgin,
and
H.B.Pakrasi
(2005).
An inquiry into protein structure and genetic disease: introducing undergraduates to bioinformatics in a large introductory course.
|
| |
Cell Biol Educ,
4,
207-220.
|
 |
|
|
|
|
 |
A.Jasaitis,
F.Rappaport,
E.Pilet,
U.Liebl,
and
M.H.Vos
(2005).
Activationless electron transfer through the hydrophobic core of cytochrome c oxidase.
|
| |
Proc Natl Acad Sci U S A,
102,
10882-10886.
|
 |
|
|
|
|
 |
A.S.Fandiño,
I.Rais,
M.Vollmer,
H.Elgass,
H.Schägger,
and
M.Karas
(2005).
LC-nanospray-MS/MS analysis of hydrophobic proteins from membrane protein complexes isolated by blue-native electrophoresis.
|
| |
J Mass Spectrom,
40,
1223-1231.
|
 |
|
|
|
|
 |
D.A.Pratt,
R.P.Pesavento,
and
W.A.van der Donk
(2005).
Model studies of the histidine-tyrosine cross-link in cytochrome C oxidase reveal the flexible substituent effect of the imidazole moiety.
|
| |
Org Lett,
7,
2735-2738.
|
 |
|
|
|
|
 |
E.Yamashita,
H.Aoyama,
M.Yao,
K.Muramoto,
K.Shinzawa-Itoh,
S.Yoshikawa,
and
T.Tsukihara
(2005).
Absolute configuration of the hydroxyfarnesylethyl group of haem A, determined by X-ray structural analysis of bovine heart cytochrome c oxidase using methods applicable at 2.8 Angstrom resolution.
|
| |
Acta Crystallogr D Biol Crystallogr,
61,
1373-1377.
|
 |
|
|
|
|
 |
H.B.Gray,
and
J.R.Winkler
(2005).
Long-range electron transfer.
|
| |
Proc Natl Acad Sci U S A,
102,
3534-3539.
|
 |
|
|
|
|
 |
H.Leonov,
and
I.T.Arkin
(2005).
A periodicity analysis of transmembrane helices.
|
| |
Bioinformatics,
21,
2604-2610.
|
 |
|
|
|
|
 |
J.G.Liu,
Y.Naruta,
and
F.Tani
(2005).
A functional model of the cytochrome c oxidase active site: unique conversion of a heme-mu-peroxo-Cu(II) intermediate into heme- superoxo/Cu(I).
|
| |
Angew Chem Int Ed Engl,
44,
1836-1840.
|
 |
|
|
|
|
 |
L.Salomonsson,
K.Faxén,
P.Adelroth,
and
P.Brzezinski
(2005).
The timing of proton migration in membrane-reconstituted cytochrome c oxidase.
|
| |
Proc Natl Acad Sci U S A,
102,
17624-17629.
|
 |
|
|
|
|
 |
M.D.Swain,
and
D.E.Benson
(2005).
Geometric preferences of crosslinked protein-derived cofactors reveal a high propensity for near-sequence pairs.
|
| |
Proteins,
59,
64-71.
|
 |
|
|
|
|
 |
M.Wikström,
C.Ribacka,
M.Molin,
L.Laakkonen,
M.Verkhovsky,
and
A.Puustinen
(2005).
Gating of proton and water transfer in the respiratory enzyme cytochrome c oxidase.
|
| |
Proc Natl Acad Sci U S A,
102,
10478-10481.
|
 |
|
|
|
|
 |
R.A.Ghiladi,
H.W.Huang,
P.Moënne-Loccoz,
J.Stasser,
N.J.Blackburn,
A.S.Woods,
R.J.Cotter,
C.D.Incarvito,
A.L.Rheingold,
and
K.D.Karlin
(2005).
Heme-copper/dioxygen adduct formation relevant to cytochrome c oxidase: spectroscopic characterization of [(6L)FeIII-(O2(2-))-CuII]+.
|
| |
J Biol Inorg Chem,
10,
63-77.
|
 |
|
|
|
|
 |
S.Hirota,
H.Okumura,
S.Kuroiwa,
N.Funasaki,
and
Y.Watanabe
(2005).
Reduction of ferricytochrome c by tyrosyltyrosylphenylalanine.
|
| |
J Biol Inorg Chem,
10,
355-363.
|
 |
|
|
|
|
 |
S.Papa
(2005).
Role of cooperative H(+)/e(-) linkage (redox bohr effect) at heme a/Cu(A) and heme a(3)/Cu(B) in the proton pump of cytochrome c oxidase.
|
| |
Biochemistry (Mosc),
70,
178-186.
|
 |
|
|
|
|
 |
S.S.Kuznetsova,
N.V.Azarkina,
T.V.Vygodina,
S.A.Siletsky,
and
A.A.Konstantinov
(2005).
Zinc ions as cytochrome C oxidase inhibitors: two sites of action.
|
| |
Biochemistry (Mosc),
70,
128-136.
|
 |
|
|
|
|
 |
T.M.Bandeiras,
M.M.Pereira,
M.Teixeira,
P.Moenne-Loccoz,
and
N.J.Blackburn
(2005).
Structure and coordination of CuB in the Acidianus ambivalens aa3 quinol oxidase heme-copper center.
|
| |
J Biol Inorg Chem,
10,
625-635.
|
 |
|
|
|
|
 |
V.M.Selaković,
M.D.Jovanović,
R.R.Mihajlović,
and
L.L.Radenović
(2005).
Dynamics of cytochrome c oxidase activity in acute ischemic stroke.
|
| |
Acta Neurol Scand,
111,
329-332.
|
 |
|
|
|
|
 |
V.Renugopalakrishnan,
M.Ortiz-Lombardía,
and
C.Verma
(2005).
Electrostatics of Cytochrome-c assemblies.
|
| |
J Mol Model,
11,
265-270.
|
 |
|
|
|
|
 |
B.L.Victor,
A.M.Baptista,
and
C.M.Soares
(2004).
Theoretical identification of proton channels in the quinol oxidase aa3 from Acidianus ambivalens.
|
| |
Biophys J,
87,
4316-4325.
|
 |
|
|
|
|
 |
C.Koutsoupakis,
T.Soulimane,
and
C.Varotsis
(2004).
Probing the Q-proton pathway of ba3-cytochrome c oxidase by time-resolved Fourier transform infrared spectroscopy.
|
| |
Biophys J,
86,
2438-2444.
|
 |
|
|
|
|
 |
H.Danielsson Thorell,
N.H.Beyer,
N.H.Heegaard,
M.Ohman,
and
T.Nilsson
(2004).
Comparison of native and recombinant chlorite dismutase from Ideonella dechloratans.
|
| |
Eur J Biochem,
271,
3539-3546.
|
 |
|
|
|
|
 |
K.B.Murray,
W.R.Taylor,
and
J.M.Thornton
(2004).
Toward the detection and validation of repeats in protein structure.
|
| |
Proteins,
57,
365-380.
|
 |
|
|
|
|
 |
K.Wagner,
N.Van Mau,
S.Boichot,
A.V.Kajava,
U.Krauss,
C.Le Grimellec,
A.Beck-Sickinger,
and
F.Heitz
(2004).
Interactions of the human calcitonin fragment 9-32 with phospholipids: a monolayer study.
|
| |
Biophys J,
87,
386-395.
|
 |
|
|
|
|
 |
M.L.Tan,
I.Balabin,
and
J.N.Onuchic
(2004).
Dynamics of electron transfer pathways in cytochrome C oxidase.
|
| |
Biophys J,
86,
1813-1819.
|
 |
|
|
|
|
 |
P.Brzezinski
(2004).
Redox-driven membrane-bound proton pumps.
|
| |
Trends Biochem Sci,
29,
380-387.
|
 |
|
|
|
|
 |
S.Braun-Sand,
M.Strajbl,
and
A.Warshel
(2004).
Studies of proton translocations in biological systems: simulating proton transport in carbonic anhydrase by EVB-based models.
|
| |
Biophys J,
87,
2221-2239.
|
 |
|
|
|
|
 |
T.I.Karu,
L.V.Pyatibrat,
and
N.I.Afanasyeva
(2004).
A novel mitochondrial signaling pathway activated by visible-to-near infrared radiation.
|
| |
Photochem Photobiol,
80,
366-372.
|
 |
|
|
|
|
 |
X.Zheng,
Y.Georgievskii,
and
A.A.Stuchebrukhov
(2004).
On the electron tunneling in molecules: a generalized orthogonalization procedure for finding tunneling orbitals.
|
| |
J Chem Phys,
121,
8680-8686.
|
 |
|
|
|
|
 |
A.Burykin,
and
A.Warshel
(2003).
What really prevents proton transport through aquaporin? Charge self-energy versus proton wire proposals.
|
| |
Biophys J,
85,
3696-3706.
|
 |
|
|
|
|
 |
E.Ching,
R.B.Gennis,
and
R.W.Larsen
(2003).
Kinetics of intramolecular electron transfer in cytochrome bo3 from Escherichia coli.
|
| |
Biophys J,
84,
2728-2733.
|
 |
|
|
|
|
 |
E.Kim,
M.E.Helton,
I.M.Wasser,
K.D.Karlin,
S.Lu,
H.W.Huang,
P.Moenne-Loccoz,
C.D.Incarvito,
A.L.Rheingold,
M.Honecker,
S.Kaderli,
and
A.D.Zuberbuhler
(2003).
Superoxo, mu-peroxo, and mu-oxo complexes from heme/O2 and heme-Cu/O2 reactivity: copper ligand influences in cytochrome c oxidase models.
|
| |
Proc Natl Acad Sci U S A,
100,
3623-3628.
|
 |
|
|
|
|
 |
H.B.Gray
(2003).
Biological inorganic chemistry at the beginning of the 21st century.
|
| |
Proc Natl Acad Sci U S A,
100,
3563-3568.
|
 |
|
|
|
|
 |
H.Imai,
and
Y.Nakagawa
(2003).
Biological significance of phospholipid hydroperoxide glutathione peroxidase (PHGPx, GPx4) in mammalian cells.
|
| |
Free Radic Biol Med,
34,
145-169.
|
 |
|
|
|
|
 |
J.A.Kornblatt,
B.C.Hill,
and
M.C.Marden
(2003).
The influence of temperature and osmolyte on the catalytic cycle of cytochrome c oxidase.
|
| |
Eur J Biochem,
270,
253-260.
|
 |
|
|
|
|
 |
J.A.Sigman,
H.K.Kim,
X.Zhao,
J.R.Carey,
and
Y.Lu
(2003).
The role of copper and protons in heme-copper oxidases: kinetic study of an engineered heme-copper center in myoglobin.
|
| |
Proc Natl Acad Sci U S A,
100,
3629-3634.
|
 |
|
|
|
|
 |
M.Milani,
P.Y.Savard,
H.Ouellet,
P.Ascenzi,
M.Guertin,
and
M.Bolognesi
(2003).
A TyrCD1/TrpG8 hydrogen bond network and a TyrB10TyrCD1 covalent link shape the heme distal site of Mycobacterium tuberculosis hemoglobin O.
|
| |
Proc Natl Acad Sci U S A,
100,
5766-5771.
|
 |
|
PDB code:
|
 |
|
|
|
|
|
 |
T.Tsukihara,
K.Shimokata,
Y.Katayama,
H.Shimada,
K.Muramoto,
H.Aoyama,
M.Mochizuki,
K.Shinzawa-Itoh,
E.Yamashita,
M.Yao,
Y.Ishimura,
and
S.Yoshikawa
(2003).
The low-spin heme of cytochrome c oxidase as the driving element of the proton-pumping process.
|
| |
Proc Natl Acad Sci U S A,
100,
15304-15309.
|
 |
|
PDB codes:
|
 |
|
|
|
|
|
 |
C.E.Cooper
(2002).
Nitric oxide and cytochrome oxidase: substrate, inhibitor or effector?
|
| |
Trends Biochem Sci,
27,
33-39.
|
 |
|
|
|
|
 |
D.Heitbrink,
H.Sigurdson,
C.Bolwien,
P.Brzezinski,
and
J.Heberle
(2002).
Transient binding of CO to Cu(B) in cytochrome c oxidase is dynamically linked to structural changes around a carboxyl group: a time-resolved step-scan Fourier transform infrared investigation.
|
| |
Biophys J,
82,
1.
|
 |
|
|
|
|
 |
F.Tomson,
J.A.Bailey,
R.B.Gennis,
C.J.Unkefer,
Z.Li,
L.A.Silks,
R.A.Martinez,
R.J.Donohoe,
R.B.Dyer,
and
W.H.Woodruff
(2002).
Direct infrared detection of the covalently ring linked His-Tyr structure in the active site of the heme-copper oxidases.
|
| |
Biochemistry,
41,
14383-14390.
|
 |
|
|
|
|
 |
L.B.Anderson,
M.Maderia,
A.J.Ouellette,
C.Putnam-Evans,
L.Higgins,
T.Krick,
M.J.MacCoss,
H.Lim,
J.R.Yates,
and
B.A.Barry
(2002).
Posttranslational modifications in the CP43 subunit of photosystem II.
|
| |
Proc Natl Acad Sci U S A,
99,
14676-14681.
|
 |
|
|
|
|
 |
P.Hellwig,
U.Pfitzner,
J.Behr,
B.Rost,
R.P.Pesavento,
W.V.Donk,
R.B.Gennis,
H.Michel,
B.Ludwig,
and
W.Mäntele
(2002).
Vibrational modes of tyrosines in cytochrome c oxidase from Paracoccus denitrificans: FTIR and electrochemical studies on Tyr-D4-labeled and on Tyr280His and Tyr35Phe mutant enzymes.
|
| |
Biochemistry,
41,
9116-9125.
|
 |
|
|
|
|
 |
Y.Watanabe
(2002).
Construction of heme enzymes: four approaches.
|
| |
Curr Opin Chem Biol,
6,
208-216.
|
 |
|
|
|
|
 |
Y.Zhen,
B.Schmidt,
U.G.Kang,
W.Antholine,
and
S.Ferguson-Miller
(2002).
Mutants of the CuA site in cytochrome c oxidase of Rhodobacter sphaeroides: I. Spectral and functional properties.
|
| |
Biochemistry,
41,
2288-2297.
|
 |
|
|
|
|
 |
B.E.Schultz,
and
S.I.Chan
(2001).
Structures and proton-pumping strategies of mitochondrial respiratory enzymes.
|
| |
Annu Rev Biophys Biomol Struct,
30,
23-65.
|
 |
|
|
|
|
 |
B.Ludwig,
E.Bender,
S.Arnold,
M.Hüttemann,
I.Lee,
and
B.Kadenbach
(2001).
Cytochrome C oxidase and the regulation of oxidative phosphorylation.
|
| |
Chembiochem,
2,
392-403.
|
 |
|
|
|
|
 |
D.Ricard,
A.Didier,
M.L'Her,
and
B.Boitrel
(2001).
Application of 3-quinolinoyl picket porphyrins to the electroreduction of dioxygen to water: mimicking the active site of cytochrome c oxidase.
|
| |
Chembiochem,
2,
144-148.
|
 |
|
|
|
|
 |
D.Ricard,
M.L'Her,
P.Richard,
and
B.Boitrel
(2001).
Iron porphyrins as models of cytochrome c oxidase.
|
| |
Chemistry,
7,
3291-3297.
|
 |
|
|
|
|
 |
E.Forte,
A.Urbani,
M.Saraste,
P.Sarti,
M.Brunori,
and
A.Giuffrè
(2001).
The cytochrome cbb3 from Pseudomonas stutzeri displays nitric oxide reductase activity.
|
| |
Eur J Biochem,
268,
6486-6491.
|
 |
|
|
|
|
 |
G.Gilderson,
A.Aagaard,
C.M.Gomes,
P.Adelroth,
M.Teixeira,
and
P.Brzezinski
(2001).
Kinetics of electron and proton transfer during O(2) reduction in cytochrome aa(3) from A. ambivalens: an enzyme lacking Glu(I-286).
|
| |
Biochim Biophys Acta,
1503,
261-270.
|
 |
|
|
|
|
 |
I.E.Scheffler
(2001).
Mitochondria make a come back.
|
| |
Adv Drug Deliv Rev,
49,
3.
|
 |
|
|
|
|
 |
I.Lee,
E.Bender,
S.Arnold,
and
B.Kadenbach
(2001).
New control of mitochondrial membrane potential and ROS formation--a hypothesis.
|
| |
Biol Chem,
382,
1629-1636.
|
 |
|
|
|
|
 |
I.Szundi,
G.L.Liao,
and
O.Einarsdóttir
(2001).
Near-infrared time-resolved optical absorption studies of the reaction of fully reduced cytochrome c oxidase with dioxygen.
|
| |
Biochemistry,
40,
2332-2339.
|
 |
|
|
|
|
 |
J.Abramson,
M.Svensson-Ek,
B.Byrne,
and
S.Iwata
(2001).
Structure of cytochrome c oxidase: a comparison of the bacterial and mitochondrial enzymes.
|
| |
Biochim Biophys Acta,
1544,
1-9.
|
 |
|
|
|
|
 |
J.E.Morgan,
M.I.Verkhovsky,
G.Palmer,
and
M.Wikström
(2001).
Role of the PR intermediate in the reaction of cytochrome c oxidase with O2.
|
| |
Biochemistry,
40,
6882-6892.
|
 |
|
|
|
|
 |
L.Xie,
and
W.A.van der Donk
(2001).
Homemade cofactors: self-processing in galactose oxidase.
|
| |
Proc Natl Acad Sci U S A,
98,
12863-12865.
|
 |
|
|
|
|
 |
M.A.Halcrow
(2001).
Chemically Modified Amino Acids in Copper Proteins That Bind or Activate Dioxygen The author acknowledges the Royal Society (London) for a University Research Fellowship.
|
| |
Angew Chem Int Ed Engl,
40,
346-349.
|
 |
|
|
|
|
 |
M.Fabian,
and
G.Palmer
(2001).
Proton involvement in the transition from the "peroxy" to the ferryl intermediate of cytochrome c oxidase.
|
| |
Biochemistry,
40,
1867-1874.
|
 |
|
|
|
|
 |
M.Fabian,
L.Skultety,
C.Brunel,
and
G.Palmer
(2001).
Cyanide stimulated dissociation of chloride from the catalytic center of oxidized cytochrome c oxidase.
|
| |
Biochemistry,
40,
6061-6069.
|
 |
|
|
|
|
 |
M.H.Vos,
G.Lipowski,
J.C.Lambry,
J.L.Martin,
and
U.Liebl
(2001).
Dynamics of nitric oxide in the active site of reduced cytochrome c oxidase aa3.
|
| |
Biochemistry,
40,
7806-7811.
|
 |
|
|
|
|
 |
M.Santana,
M.M.Pereira,
N.P.Elias,
C.M.Soares,
and
M.Teixeira
(2001).
Gene cluster of Rhodothermus marinus high-potential iron-sulfur Protein: oxygen oxidoreductase, a caa(3)-type oxidase belonging to the superfamily of heme-copper oxidases.
|
| |
J Bacteriol,
183,
687-699.
|
 |
|
|
|
|
 |
N.Lehnert,
S.D.George,
and
E.I.Solomon
(2001).
Recent advances in bioinorganic spectroscopy.
|
| |
Curr Opin Chem Biol,
5,
176-187.
|
 |
|
|
|
|
 |
P.R.Rich,
and
J.Breton
(2001).
FTIR studies of the CO and cyanide adducts of fully reduced bovine cytochrome c oxidase.
|
| |
Biochemistry,
40,
6441-6449.
|
 |
|
|
|
|
 |
S.Akashi,
and
K.Takio
(2001).
Structure of melittin bound to phospholipid micelles studied using hydrogen-deuterium exchange and electrospray ionization Fourier transform ion cyclotron resonance mass spectrometry.
|
| |
J Am Soc Mass Spectrom,
12,
1247-1253.
|
 |
|
|
|
|
 |
S.Dastmalchi,
M.B.Morris,
and
W.B.Church
(2001).
Modeling of the structural features of integral-membrane proteins reverse-environment prediction of integral membrane protein structure (REPIMPS).
|
| |
Protein Sci,
10,
1529-1538.
|
 |
|
|
|
|
 |
S.J.Lee,
E.Yamashita,
T.Abe,
Y.Fukumoto,
T.Tsukihara,
K.Shinzawa-Itoh,
H.Ueda,
and
S.Yoshikawa
(2001).
Intermonomer interactions in dimer of bovine heart cytochrome c oxidase.
|
| |
Acta Crystallogr D Biol Crystallogr,
57,
941-947.
|
 |
|
|
|
|
 |
Suharti,
M.J.Strampraad,
I.Schröder,
and
S.de Vries
(2001).
A novel copper A containing menaquinol NO reductase from Bacillus azotoformans.
|
| |
Biochemistry,
40,
2632-2639.
|
 |
|
|
|
|
 |
T.K.Das,
F.L.Tomson,
R.B.Gennis,
M.Gordon,
and
D.L.Rousseau
(2001).
pH-dependent structural changes at the Heme-Copper binuclear center of cytochrome c oxidase.
|
| |
Biophys J,
80,
2039-2045.
|
 |
|
|
|
|
 |
V.Sampson,
and
T.Alleyne
(2001).
Cytochrome c/cytochrome c oxidase interaction. Direct structural evidence for conformational changes during enzyme turnover.
|
| |
Eur J Biochem,
268,
6534-6544.
|
 |
|
|
|
|
 |
A.Giuffrè,
M.C.Barone,
D.Mastronicola,
E.D'Itri,
P.Sarti,
and
M.Brunori
(2000).
Reaction of nitric oxide with the turnover intermediates of cytochrome c oxidase: reaction pathway and functional effects.
|
| |
Biochemistry,
39,
15446-15453.
|
 |
|
|
|
|
 |
B.Kadenbach,
M.Hüttemann,
S.Arnold,
I.Lee,
and
E.Bender
(2000).
Mitochondrial energy metabolism is regulated via nuclear-coded subunits of cytochrome c oxidase.
|
| |
Free Radic Biol Med,
29,
211-221.
|
 |
|
|
|
|
 |
B.Schwartz,
J.E.Dove,
and
J.P.Klinman
(2000).
Kinetic analysis of oxygen utilization during cofactor biogenesis in a copper-containing amine oxidase from yeast.
|
| |
Biochemistry,
39,
3699-3707.
|
 |
|
|
|
|
 |
C.Backgren,
G.Hummer,
M.Wikström,
and
A.Puustinen
(2000).
Proton translocation by cytochrome c oxidase can take place without the conserved glutamic acid in subunit I.
|
| |
Biochemistry,
39,
7863-7867.
|
 |
|
|
|
|
 |
D.J.Hunter,
V.S.Oganesyan,
J.C.Salerno,
C.S.Butler,
W.J.Ingledew,
and
A.J.Thomson
(2000).
Angular dependences of perpendicular and parallel mode electron paramagnetic resonance of oxidized beef heart cytochrome c oxidase.
|
| |
Biophys J,
78,
439-450.
|
 |
|
|
|
|
 |
D.Zaslavsky,
and
R.B.Gennis
(2000).
Proton pumping by cytochrome oxidase: progress, problems and postulates.
|
| |
Biochim Biophys Acta,
1458,
164-179.
|
 |
|
|
|
|
 |
H.L.Axelrod,
E.C.Abresch,
M.L.Paddock,
M.Y.Okamura,
and
G.Feher
(2000).
Determination of the binding sites of the proton transfer inhibitors Cd2+ and Zn2+ in bacterial reaction centers.
|
| |
Proc Natl Acad Sci U S A,
97,
1542-1547.
|
 |
|
PDB codes:
|
 |
|
|
|
|
|
 |
H.M.Lee,
T.K.Das,
D.L.Rousseau,
D.Mills,
S.Ferguson-Miller,
and
R.B.Gennis
(2000).
Mutations in the putative H-channel in the cytochrome c oxidase from Rhodobacter sphaeroides show that this channel is not important for proton conduction but reveal modulation of the properties of heme a.
|
| |
Biochemistry,
39,
2989-2996.
|
 |
|
|
|
|
 |
J.Behr,
H.Michel,
W.Mäntele,
and
P.Hellwig
(2000).
Functional properties of the heme propionates in cytochrome c oxidase from Paracoccus denitrificans. Evidence from FTIR difference spectroscopy and site-directed mutagenesis.
|
| |
Biochemistry,
39,
1356-1363.
|
 |
|
|
|
|
 |
J.L.Popot,
and
D.M.Engelman
(2000).
Helical membrane protein folding, stability, and evolution.
|
| |
Annu Rev Biochem,
69,
881-922.
|
 |
|
|
|
|
 |
K.Kobayashi,
S.Tagawa,
and
T.Mogi
(2000).
Transient formation of ubisemiquinone radical and subsequent electron transfer process in the Escherichia coli cytochrome bo.
|
| |
Biochemistry,
39,
15620-15625.
|
 |
|
|
|
|
 |
M.H.Vos,
V.B.Borisov,
U.Liebl,
J.L.Martin,
and
A.A.Konstantinov
(2000).
Femtosecond resolution of ligand-heme interactions in the high-affinity quinol oxidase bd: A di-heme active site?
|
| |
Proc Natl Acad Sci U S A,
97,
1554-1559.
|
 |
|
|
|
|
 |
M.J.Fei,
E.Yamashita,
N.Inoue,
M.Yao,
H.Yamaguchi,
T.Tsukihara,
K.Shinzawa-Itoh,
R.Nakashima,
and
S.Yoshikawa
(2000).
X-ray structure of azide-bound fully oxidized cytochrome c oxidase from bovine heart at 2.9 A resolution.
|
| |
Acta Crystallogr D Biol Crystallogr,
56,
529-535.
|
 |
|
|
|
|
 |
M.Karpefors,
P.Adelroth,
A.Namslauer,
Y.Zhen,
and
P.Brzezinski
(2000).
Formation of the "peroxy" intermediate in cytochrome c oxidase is associated with internal proton/hydrogen transfer.
|
| |
Biochemistry,
39,
14664-14669.
|
 |
|
|
|
|
 |
M.R.Bratton,
L.Hiser,
W.E.Antholine,
C.Hoganson,
and
J.P.Hosler
(2000).
Identification of the structural subunits required for formation of the metal centers in subunit I of cytochrome c oxidase of Rhodobacter sphaeroides.
|
| |
Biochemistry,
39,
12989-12995.
|
 |
|
|
|
|
 |
M.Wikström
(2000).
Mechanism of proton translocation by cytochrome c oxidase: a new four-stroke histidine cycle.
|
| |
Biochim Biophys Acta,
1458,
188-198.
|
 |
|
|
|
|
 |
M.Wikström,
A.Jasaitis,
C.Backgren,
A.Puustinen,
and
M.I.Verkhovsky
(2000).
The role of the D- and K-pathways of proton transfer in the function of the haem-copper oxidases.
|
| |
Biochim Biophys Acta,
1459,
514-520.
|
 |
|
|
|
|
 |
N.Capitanio,
G.Capitanio,
M.Minuto,
E.De Nitto,
L.L.Palese,
P.Nicholls,
and
S.Papa
(2000).
Coupling of electron transfer with proton transfer at heme a and Cu(A) (redox Bohr effects) in cytochrome c oxidase. Studies with the carbon monoxide inhibited enzyme.
|
| |
Biochemistry,
39,
6373-6379.
|
 |
|
|
|
|
 |
N.M.Okeley,
and
W.A.van der Donk
(2000).
Novel cofactors via post-translational modifications of enzyme active sites.
|
| |
Chem Biol,
7,
R159-R171.
|
 |
|
|
|
|
 |
O.Farver,
O.Einarsdóttir,
and
I.Pecht
(2000).
Electron transfer rates and equilibrium within cytochrome c oxidase.
|
| |
Eur J Biochem,
267,
950-954.
|
 |
|
|
|
|
 |
R.J.Debus,
K.A.Campbell,
D.P.Pham,
A.M.Hays,
and
R.D.Britt
(2000).
Glutamate 189 of the D1 polypeptide modulates the magnetic and redox properties of the manganese cluster and tyrosine Y(Z) in photosystem II.
|
| |
Biochemistry,
39,
6275-6287.
|
 |
|
|
|
|
 |
S.E.Rigby,
S.Jünemann,
P.R.Rich,
and
P.Heathcote
(2000).
Reaction of bovine cytochrome c oxidase with hydrogen peroxide produces a tryptophan cation radical and a porphyrin cation radical.
|
| |
Biochemistry,
39,
5921-5928.
|
 |
|
|
|
|
 |
S.Jünemann,
P.Heathcote,
and
P.R.Rich
(2000).
The reactions of hydrogen peroxide with bovine cytochrome c oxidase.
|
| |
Biochim Biophys Acta,
1456,
56-66.
|
 |
|
|
|
|
 |
T.K.Das,
and
S.Mazumdar
(2000).
Redox-linked conformational changes in bovine heart cytochrome c oxidase: picosecond time-resolved fluorescence studies of cyanide complex.
|
| |
Biopolymers,
57,
316-322.
|
 |
|
|
|
|
 |
T.Páli,
J.H.Kleinschmidt,
G.L.Powell,
and
D.Marsh
(2000).
Nonlinear electron paramagnetic resonance studies of the interaction of cytochrome c oxidase with spin-labeled lipids in gel-phase membranes.
|
| |
Biochemistry,
39,
2355-2361.
|
 |
|
|
|
|
 |
T.Sjögren,
M.Svensson-Ek,
J.Hajdu,
and
P.Brzezinski
(2000).
Proton-coupled structural changes upon binding of carbon monoxide to cytochrome cd1: a combined flash photolysis and X-ray crystallography study.
|
| |
Biochemistry,
39,
10967-10974.
|
 |
|
PDB code:
|
 |
|
|
|
|
|
 |
T.Soulimane,
G.Buse,
G.P.Bourenkov,
H.D.Bartunik,
R.Huber,
and
M.E.Than
(2000).
Structure and mechanism of the aberrant ba(3)-cytochrome c oxidase from thermus thermophilus.
|
| |
EMBO J,
19,
1766-1776.
|
 |
|
PDB code:
|
 |
|
|
|
|
|
 |
T.Soulimane,
M.E.Than,
M.Dewor,
R.Huber,
and
G.Buse
(2000).
Primary structure of a novel subunit in ba3-cytochrome oxidase from Thermus thermophilus.
|
| |
Protein Sci,
9,
2068-2073.
|
 |
|
|
|
|
 |
T.Uchida,
T.Mogi,
and
T.Kitagawa
(2000).
Resonance raman studies of oxo intermediates in the reaction of pulsed cytochrome bo with hydrogen peroxide.
|
| |
Biochemistry,
39,
6669-6678.
|
 |
|
|
|
|
 |
U.Pfitzner,
K.Hoffmeier,
A.Harrenga,
A.Kannt,
H.Michel,
E.Bamberg,
O.M.Richter,
and
B.Ludwig
(2000).
Tracing the D-pathway in reconstituted site-directed mutants of cytochrome c oxidase from Paracoccus denitrificans.
|
| |
Biochemistry,
39,
6756-6762.
|
 |
|
|
|
|
 |
A.Giuffrè,
E.Forte,
G.Antonini,
E.D'Itri,
M.Brunori,
T.Soulimane,
and
G.Buse
(1999).
Kinetic properties of ba3 oxidase from Thermus thermophilus: effect of temperature.
|
| |
Biochemistry,
38,
1057-1065.
|
 |
|
|
|
|
 |
A.Giuffrè,
G.Stubauer,
P.Sarti,
M.Brunori,
W.G.Zumft,
G.Buse,
and
T.Soulimane
(1999).
The heme-copper oxidases of Thermus thermophilus catalyze the reduction of nitric oxide: evolutionary implications.
|
| |
Proc Natl Acad Sci U S A,
96,
14718-14723.
|
 |
|
|
|
|
 |
D.Zaslavsky,
I.A.Smirnova,
P.Brzezinski,
K.Shinzawa-Itoh,
S.Yoshikawa,
and
R.B.Gennis
(1999).
Examination of the reaction of fully reduced cytochrome oxidase with hydrogen peroxide by flow-flash spectroscopy.
|
| |
Biochemistry,
38,
16016-16023.
|
 |
|
|
|
|
 |
F.MacMillan,
A.Kannt,
J.Behr,
T.Prisner,
and
H.Michel
(1999).
Direct evidence for a tyrosine radical in the reaction of cytochrome c oxidase with hydrogen peroxide.
|
| |
Biochemistry,
38,
9179-9184.
|
 |
|
|
|
|
 |
G.Buse,
T.Soulimane,
M.Dewor,
H.E.Meyer,
and
M.Blüggel
(1999).
Evidence for a copper-coordinated histidine-tyrosine cross-link in the active site of cytochrome oxidase.
|
| |
Protein Sci,
8,
985-990.
|
 |
|
|
|
|
 |
G.T.Babcock
(1999).
How oxygen is activated and reduced in respiration.
|
| |
Proc Natl Acad Sci U S A,
96,
12971-12973.
|
 |
|
|
|
|
 |
G.Villani,
N.Capitanio,
A.Bizzoca,
L.L.Palese,
V.Carlino,
M.Tattoli,
P.Glaser,
A.Danchin,
and
S.Papa
(1999).
Effects of site-directed mutagenesis of protolytic residues in subunit I of Bacillus subtilis aa3-600 quinol oxidase. Role of lysine 304 in proton translocation.
|
| |
Biochemistry,
38,
2287-2294.
|
 |
|
|
|
|
 |
H.C.Liang,
M.Dahan,
and
K.D.Karlin
(1999).
Dioxygen-activating bio-inorganic model complexes.
|
| |
Curr Opin Chem Biol,
3,
168-175.
|
 |
|
|
|
|
 |
H.Michel
(1999).
Cytochrome c oxidase: catalytic cycle and mechanisms of proton pumping--a discussion.
|
| |
Biochemistry,
38,
15129-15140.
|
 |
|
|
|
|
 |
I.A.Smirnova,
P.Adelroth,
R.B.Gennis,
and
P.Brzezinski
(1999).
Aspartate-132 in cytochrome c oxidase from Rhodobacter sphaeroides is involved in a two-step proton transfer during oxo-ferryl formation.
|
| |
Biochemistry,
38,
6826-6833.
|
 |
|
|
|
|
 |
J.P.Osborne,
N.J.Cosper,
C.M.Stälhandske,
R.A.Scott,
J.O.Alben,
and
R.B.Gennis
(1999).
Cu XAS shows a change in the ligation of CuB upon reduction of cytochrome bo3 from Escherichia coli.
|
| |
Biochemistry,
38,
4526-4532.
|
 |
|
|
|
|
 |
M.Fabian,
and
G.Palmer
(1999).
Redox state of peroxy and ferryl intermediates in cytochrome c oxidase catalysis.
|
| |
Biochemistry,
38,
6270-6275.
|
 |
|
|
|
|
 |
M.Fabian,
W.W.Wong,
R.B.Gennis,
and
G.Palmer
(1999).
Mass spectrometric determination of dioxygen bond splitting in the "peroxy" intermediate of cytochrome c oxidase.
|
| |
Proc Natl Acad Sci U S A,
96,
13114-13117.
|
 |
|
|
|
|
 |
M.H.Vos,
and
J.L.Martin
(1999).
Femtosecond processes in proteins.
|
| |
Biochim Biophys Acta,
1411,
1.
|
 |
|
|
|
|
 |
M.Lübben,
A.Prutsch,
B.Mamat,
and
K.Gerwert
(1999).
Electron transfer induces side-chain conformational changes of glutamate-286 from cytochrome bo3.
|
| |
Biochemistry,
38,
2048-2056.
|
 |
|
|
|
|
 |
M.R.Bratton,
M.A.Pressler,
and
J.P.Hosler
(1999).
Suicide inactivation of cytochrome c oxidase: catalytic turnover in the absence of subunit III alters the active site.
|
| |
Biochemistry,
38,
16236-16245.
|
 |
|
|
|
|
 |
M.Ralle,
M.L.Verkhovskaya,
J.E.Morgan,
M.I.Verkhovsky,
M.Wikström,
and
N.J.Blackburn
(1999).
Coordination of CuB in reduced and CO-liganded states of cytochrome bo3 from Escherichia coli. Is chloride ion a cofactor?
|
| |
Biochemistry,
38,
7185-7194.
|
 |
|
|
|
|
 |
P.Hellwig,
S.Grzybek,
J.Behr,
B.Ludwig,
H.Michel,
and
W.Mäntele
(1999).
Electrochemical and ultraviolet/visible/infrared spectroscopic analysis of heme a and a3 redox reactions in the cytochrome c oxidase from Paracoccus denitrificans: separation of heme a and a3 contributions and assignment of vibrational modes.
|
| |
Biochemistry,
38,
1685-1694.
|
 |
|
|
|
|
 |
S.Hirota,
T.Iwamoto,
K.Tanizawa,
O.Adachi,
and
O.Yamauchi
(1999).
Spectroscopic characterization of carbon monoxide complexes generated for copper/topa quinone-containing amine oxidases.
|
| |
Biochemistry,
38,
14256-14263.
|
 |
|
|
|
|
 |
S.Paula,
A.Sucheta,
I.Szundi,
and
O.Einarsdóttir
(1999).
Proton and electron transfer during the reduction of molecular oxygen by fully reduced cytochrome c oxidase: a flow-flash investigation using optical multichannel detection.
|
| |
Biochemistry,
38,
3025-3033.
|
 |
|
|
|
|
 |
S.Siletsky,
A.D.Kaulen,
and
A.A.Konstantinov
(1999).
Resolution of electrogenic steps coupled to conversion of cytochrome c oxidase from the peroxy to the ferryl-oxo state.
|
| |
Biochemistry,
38,
4853-4861.
|
 |
|
|
|
|
 |
T.K.Das,
C.M.Gomes,
M.Teixeira,
and
D.L.Rousseau
(1999).
Redox-linked transient deprotonation at the binuclear site in the aa(3)-type quinol oxidase from Acidianus ambivalens: implications for proton translocation.
|
| |
Proc Natl Acad Sci U S A,
96,
9591-9596.
|
 |
|
|
|
|
 |
T.K.Das,
M.Couture,
H.C.Lee,
J.Peisach,
D.L.Rousseau,
B.A.Wittenberg,
J.B.Wittenberg,
and
M.Guertin
(1999).
Identification of the ligands to the ferric heme of Chlamydomonas chloroplast hemoglobin: evidence for ligation of tyrosine-63 (B10) to the heme.
|
| |
Biochemistry,
38,
15360-15368.
|
 |
|
|
|
|
 |
T.L.Poulos,
H.Li,
and
C.S.Raman
(1999).
Heme-mediated oxygen activation in biology: cytochrome c oxidase and nitric oxide synthase.
|
| |
Curr Opin Chem Biol,
3,
131-137.
|
 |
|
|
|
|
 |
A.Sucheta,
I.Szundi,
and
O.Einarsdóttir
(1998).
Intermediates in the reaction of fully reduced cytochrome c oxidase with dioxygen.
|
| |
Biochemistry,
37,
17905-17914.
|
 |
|
|
|
|
 |
H.Michel
(1998).
The mechanism of proton pumping by cytochrome c oxidasex127e comments]
|
| |
Proc Natl Acad Sci U S A,
95,
12819-12824.
|
 |
|
|
|
|
 |
K.M.Broekemeier,
C.K.Klocek,
and
D.R.Pfeiffer
(1998).
Proton selective substate of the mitochondrial permeability transition pore: regulation by the redox state of the electron transport chain.
|
| |
Biochemistry,
37,
13059-13065.
|
 |
|
|
|
|
 |
M.Karpefors,
P.Adelroth,
Y.Zhen,
S.Ferguson-Miller,
and
P.Brzezinski
(1998).
Proton uptake controls electron transfer in cytochrome c oxidase.
|
| |
Proc Natl Acad Sci U S A,
95,
13606-13611.
|
 |
|
|
|
|
 |
M.V.Ponamarev,
and
W.A.Cramer
(1998).
Perturbation of the internal water chain in cytochrome f of oxygenic photosynthesis: loss of the concerted reduction of cytochromes f and b6.
|
| |
Biochemistry,
37,
17199-17208.
|
 |
|
|
|
|
 |
R.B.Gennis
(1998).
How does cytochrome oxidase pump protons?
|
| |
Proc Natl Acad Sci U S A,
95,
12747-12749.
|
 |
|
|
|
|
 |
S.Karlin,
Z.Y.Zhu,
and
K.D.Karlin
(1998).
Extended metal environments of cytochrome c oxidase structures.
|
| |
Biochemistry,
37,
17726-17734.
|
 |
|
|
|
|
 |
S.Papa,
N.Capitanio,
G.Villani,
G.Capitanio,
A.Bizzoca,
L.L.Palese,
V.Carlino,
and
E.De Nitto
(1998).
Cooperative coupling and role of heme a in the proton pump of heme-copper oxidases.
|
| |
Biochimie,
80,
821-836.
|
 |
|
|
|
|
 |
T.K.Das,
C.Pecoraro,
F.L.Tomson,
R.B.Gennis,
and
D.L.Rousseau
(1998).
The post-translational modification in cytochrome c oxidase is required to establish a functional environment of the catalytic site.
|
| |
Biochemistry,
37,
14471-14476.
|
 |
|
|
|
|
 |
U.Ermler,
W.Grabarse,
S.Shima,
M.Goubeaud,
and
R.K.Thauer
(1998).
Active sites of transition-metal enzymes with a focus on nickel.
|
| |
Curr Opin Struct Biol,
8,
749-758.
|
 |
|
 |
 |
|
The most recent references are shown first.
Citation data come partly from CiteXplore and partly
from an automated harvesting procedure. Note that this is likely to be
only a partial list as not all journals are covered by
either method. However, we are continually building up the citation data
so more and more references will be included with time.
Where a reference describes a PDB structure, the PDB
codes are
shown on the right.
|

| |

