 |
PDBsum entry 1o2e

|

|

|
|
 |
Contents |
 |
|
|

|

|
|
|
|
|
|
|
|
|
|
* Residue conservation analysis
|
|
|
|
 |
|
|
 |
 |
 |
 |
Enzyme class:
|
 |
E.C.3.1.1.4
- phospholipase A2.
|
|
 |
 |
 |
 |
 |
Reaction:
|
 |
a 1,2-diacyl-sn-glycero-3-phosphocholine + H2O = a 1-acyl-sn-glycero-3- phosphocholine + a fatty acid + H+
|
 |
 |
 |
 |
 |
 1,2-diacyl-sn-glycero-3-phosphocholine
1,2-diacyl-sn-glycero-3-phosphocholine
|
+
|
H2O
|
=
|
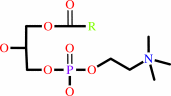 1-acyl-sn-glycero-3- phosphocholine
1-acyl-sn-glycero-3- phosphocholine
|
+
|
 fatty acid
fatty acid
|
+
|
H(+)
|
|
 |
 |
 |
 |
 |
 |
 |
 |
Cofactor:
|
 |
Ca(2+)
|
 |
 |
 |
 |
 |
 |
 |
|
Molecule diagrams generated from .mol files obtained from the
KEGG ftp site
|
|
 |
 |
 |
 |
 |
 |
 |
 |
 |
 |
 |
 |
 |
 |
 |

|

|

|
| |
|
|
| |
|
DOI no:
|
J Mol Biol
333:367-376
(2003)
|
|
PubMed id:
|
|
|
|

|
| |
|
Crystal structures of the free and anisic acid bound triple mutant of phospholipase A2.
|
|
K.Sekar,
S.Vaijayanthi Mala,
M.Yogavel,
D.Velmurugan,
M.J.Poi,
B.S.Vishwanath,
T.V.Gowda,
A.A.Jeyaprakash,
M.D.Tsai.
|
|
|

|
| |
ABSTRACT
|
|

|
| |

|
Phospholipase A2 catalyses the hydrolysis of the ester bond of
3-sn-phosphoglycerides. Here, we report the crystal structures of the free and
anisic acid-bound triple mutant (K53,56,120M) of bovine pancreatic phospholipase
A2. In the bound triple mutant structure, the small organic molecule p-anisic
acid is found in the active site, and one of the carboxylate oxygen atoms is
coordinated to the functionally important primary calcium ion. The other
carboxylate oxygen atom is hydrogen bonded to the phenolic hydroxyl group of
Tyr69. In addition, the bound anisic acid molecule replaces one of the
functionally important water molecules in the active site. The residues 60-70,
which are in a loop (surface loop), are disordered in most of the bovine
pancreatic phospholipase A2 structures. It is interesting to note that these
residues are ordered in the bound triple mutant structure but are disordered in
the free triple mutant structure. The organic crystallization ingredient
2-methyl-2,4-pentanediol is found near the active site of the free triple mutant
structure. The overall tertiary folding and stereochemical parameters for the
final models of the free and anisic acid-bound triple mutant are virtually
identical.

|

|
|
|

|
| |
Selected figure(s)
|
|

|
| |
 |
 |

|
 |

|
 |
Figure 1.
Figure 1. The backbone tracing of the free triple mutant.
The surface loop residues (60-70) and the calcium-binding loop
(27-34) are indicated with the residue numbers at the beginning
as well as at the ends. This Figure was produced using the
program MOLSCRIPT.[44.]
|
 |
Figure 4.
Figure 4. Stereo view of the omit electron density map
contoured at the 1.0s level showing the electron density for the
anisic acid molecule in the bound triple mutant structure. In
addition, the only water molecule hydrogen bonded to Nd1 of
His48 is shown and it is 0.49 Å below the plane of the
histidine ring.
|
 |
|
|

|
| |
The above figures are
reprinted
by permission from Elsevier:
J Mol Biol
(2003,
333,
367-376)
copyright 2003.
|
|
| |
Figures were
selected
by an automated process.
|
|

|

|
|
 |
 |
|
 |
 |
 |
 |
 |
 |
 |
 |
 |
|
Literature references that cite this PDB file's key reference
|
|
 |
| |
PubMed id
|
 |
Reference
|
 |
|
|
|
 |
K.Sekar,
D.Gayathri,
D.Velmurugan,
J.Jeyakanthan,
T.Yamane,
M.J.Poi,
and
M.D.Tsai
(2006).
Third calcium ion found in an inhibitor-bound phospholipase A2.
|
| |
Acta Crystallogr D Biol Crystallogr,
62,
392-397.
|
 |
|
PDB code:
|
 |
|
|
|
|
|
 |
K.Sekar,
M.Yogavel,
D.Gayathri,
D.Velmurugan,
R.Krishna,
M.J.Poi,
Z.Dauter,
M.Dauter,
and
M.D.Tsai
(2006).
Atomic resolution structure of the double mutant (K53,56M) of bovine pancreatic phospholipase A2.
|
| |
Acta Crystallogr Sect F Struct Biol Cryst Commun,
62,
1-5.
|
 |
|
|
|
|
 |
K.Sekar,
M.Yogavel,
S.P.Kanaujia,
A.Sharma,
D.Velmurugan,
M.J.Poi,
Z.Dauter,
and
M.D.Tsai
(2006).
Suggestive evidence for the involvement of the second calcium and surface loop in interfacial binding: monoclinic and trigonal crystal structures of a quadruple mutant of phospholipase A2.
|
| |
Acta Crystallogr D Biol Crystallogr,
62,
717-724.
|
 |
|
PDB codes:
|
 |
|
|
|
|
|
 |
N.Singh,
T.Jabeen,
A.Pal,
S.Sharma,
M.Perbandt,
C.Betzel,
and
T.P.Singh
(2006).
Crystal structures of the complexes of a group IIA phospholipase A2 with two natural anti-inflammatory agents, anisic acid, and atropine reveal a similar mode of binding.
|
| |
Proteins,
64,
89.
|
 |
|
PDB codes:
|
 |
|
|
|
|
|
 |
K.Sekar,
V.Rajakannan,
D.Gayathri,
D.Velmurugan,
M.J.Poi,
M.Dauter,
Z.Dauter,
and
M.D.Tsai
(2005).
Atomic resolution (0.97 A) structure of the triple mutant (K53,56,121M) of bovine pancreatic phospholipase A2.
|
| |
Acta Crystallogr Sect F Struct Biol Cryst Commun,
61,
3-7.
|
 |
|
PDB codes:
|
 |
|
|
|
|
|
 |
T.Jabeen,
N.Singh,
R.K.Singh,
S.Sharma,
R.K.Somvanshi,
S.Dey,
and
T.P.Singh
(2005).
Non-steroidal anti-inflammatory drugs as potent inhibitors of phospholipase A2: structure of the complex of phospholipase A2 with niflumic acid at 2.5 Angstroms resolution.
|
| |
Acta Crystallogr D Biol Crystallogr,
61,
1579-1586.
|
 |
|
PDB code:
|
 |
|
|
|
|
|
 |
K.Sekar,
V.Rajakannan,
D.Velmurugan,
T.Yamane,
R.Thirumurugan,
M.Dauter,
and
Z.Dauter
(2004).
A redetermination of the structure of the triple mutant (K53,56,120M) of phospholipase A2 at 1.6 A resolution using sulfur-SAS at 1.54 A wavelength.
|
| |
Acta Crystallogr D Biol Crystallogr,
60,
1586-1590.
|
 |
|
PDB code:
|
 |
|
|
 |
 |
|
The most recent references are shown first.
Citation data come partly from CiteXplore and partly
from an automated harvesting procedure. Note that this is likely to be
only a partial list as not all journals are covered by
either method. However, we are continually building up the citation data
so more and more references will be included with time.
Where a reference describes a PDB structure, the PDB
code is
shown on the right.
|
 |

