 |
PDBsum entry 1nwc

|
|
|

|
 |

|

|

|

|

|

|

|

|

|

|

|

|

|

|
 |

|

|

|
 |
 |
 |
 |
 |
 |
 |
 |
 |
 |
 |
 |
 |
 |
 |
 |
 |
 |

|

|

|

|

|

|

|

|

|
|
Oxidoreductase
|
PDB id
|
|

|

|
1nwc
|

|

|

|

|

|

|

|

|

|

|

|

|

|

|

|

|

|

|

|
|
 |
Contents |
 |
|
|

|

|
|
|
|
|
|
* Residue conservation analysis
|
|
|
|
 |
|
|
 |
 |
 |
 |
Enzyme class:
|
 |
E.C.1.2.1.11
- aspartate-semialdehyde dehydrogenase.
|
|
 |
 |
 |
 |
 |

Pathway:
|
 |
Lysine biosynthesis (early stages)
|
 |
 |
 |
 |
 |
Reaction:
|
 |
L-aspartate 4-semialdehyde + phosphate + NADP+ = 4-phospho-L-aspartate + NADPH + H+
|
 |
 |
 |
 |
 |
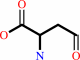 L-aspartate 4-semialdehyde
L-aspartate 4-semialdehyde
|
+
|
 phosphate
phosphate
|
+
|
 NADP(+)
NADP(+)
|
=
|
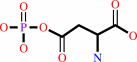 4-phospho-L-aspartate
4-phospho-L-aspartate
|
+
|
 NADPH
NADPH
|
+
|
H(+)
|
|
 |
 |
 |
 |
 |
 |
 |
 |
 |
 |
|
Molecule diagrams generated from .mol files obtained from the
KEGG ftp site
|
|
 |
 |
 |
 |
 |
 |
 |
 |
 |
 |
 |
 |
 |
 |
 |

|

|

|
| |
|
|
| |
|
DOI no:
|
Proc Natl Acad Sci U S A
100:12613-12617
(2003)
|
|
PubMed id:
|
|
|
|

|
| |
|
Capture of an intermediate in the catalytic cycle of L-aspartate-beta-semialdehyde dehydrogenase.
|
|
J.Blanco,
R.A.Moore,
R.E.Viola.
|
|
|

|
| |
ABSTRACT
|
|

|
| |

|
The structural analysis of an enzymatic reaction intermediate affords a unique
opportunity to study a catalytic mechanism in extraordinary detail. Here we
present the structure of a tetrahedral intermediate in the catalytic cycle of
aspartate-beta-semialdehyde dehydrogenase (ASADH) from Haemophilus influenzae at
2.0-A resolution. ASADH is not found in humans, yet its catalytic activity is
required for the biosynthesis of essential amino acids in plants and
microorganisms. Diaminopimelic acid, also formed by this enzymatic pathway, is
an integral component of bacterial cell walls, thus making ASADH an attractive
target for the development of new antibiotics. This enzyme is able to capture
the substrates aspartate-beta-semialdehyde and phosphate as an active complex
that does not complete the catalytic cycle in the absence of NADP. A distinctive
binding pocket in which the hemithioacetal oxygen of the bound substrate is
stabilized by interaction with a backbone amide group dictates the R
stereochemistry of the tetrahedral intermediate. This pocket, reminiscent of the
oxyanion hole found in serine proteases, is completed through hydrogen bonding
to the bound phosphate substrate.

|

|
|
|

|
| |
Selected figure(s)
|
|

|
| |
 |
 |

|
 |

|
 |
Figure 4.
Fig. 4. Schematic representation of the ASADH active site
with a bound hemithioacetal reaction intermediate. Interactions
between ASA and phosphate and key active site residues are shown.
|
 |
Figure 6.
Fig. 6. Proposed mechanism for the catalytic cycle of ASADH
shown in reverse biological direction. (A) Tetrahedral
intermediate derived from reaction with ASA. (B) Proposed acyl
intermediate with trigonal planar geometry. (C) Second
tetrahedral intermediate with covalently bound aspartyl
phosphate.
|
 |
|
|
| |
Figures were
selected
by an automated process.
|
|

|

|
|
 |
 |
|
 |
 |
 |
 |
 |
 |
 |
 |
 |
|
Literature references that cite this PDB file's key reference
|
|
 |
| |
PubMed id
|
 |
Reference
|
 |
|
|
|
 |
A.S.Evitt,
and
R.J.Cox
(2011).
Synthesis and evaluation of conformationally restricted inhibitors of aspartate semialdehyde dehydrogenase.
|
| |
Mol Biosyst,
7,
1564-1575.
|
 |
|
|
|
|
 |
I.C.Chen,
W.D.Lin,
S.K.Hsu,
V.Thiruvengadam,
and
W.H.Hsu
(2009).
Isolation and characterization of a novel lysine racemase from a soil metagenomic library.
|
| |
Appl Environ Microbiol,
75,
5161-5166.
|
 |
|
|
|
|
 |
A.Singh,
H.R.Kushwaha,
and
P.Sharma
(2008).
Molecular modelling and comparative structural account of aspartyl beta-semialdehyde dehydrogenase of Mycobacterium tuberculosis (H37Rv).
|
| |
J Mol Model,
14,
249-263.
|
 |
|
|
|
|
 |
R.E.Viola,
X.Liu,
J.F.Ohren,
and
C.R.Faehnle
(2008).
The structure of a redundant enzyme: a second isoform of aspartate beta-semialdehyde dehydrogenase in Vibrio cholerae.
|
| |
Acta Crystallogr D Biol Crystallogr,
64,
321-330.
|
 |
|
PDB codes:
|
 |
|
|
|
|
|
 |
R.Vyas,
V.Kumar,
S.Panjikar,
S.Karthikeyan,
K.V.Kishan,
R.Tewari,
and
M.S.Weiss
(2008).
Purification, crystallization and preliminary X-ray diffraction analysis of aspartate semialdehyde dehydrogenase (Rv3708c) from Mycobacterium tuberculosis.
|
| |
Acta Crystallogr Sect F Struct Biol Cryst Commun,
64,
167-170.
|
 |
|
|
|
|
 |
C.A.Hutton,
M.A.Perugini,
and
J.A.Gerrard
(2007).
Inhibition of lysine biosynthesis: an evolving antibiotic strategy.
|
| |
Mol Biosyst,
3,
458-465.
|
 |
|
|
|
|
 |
R.J.Cox,
J.S.Gibson,
and
A.T.Hadfield
(2005).
Design, synthesis and analysis of inhibitors of bacterial aspartate semialdehyde dehydrogenase.
|
| |
Chembiochem,
6,
2255-2260.
|
 |
|
|
|
|
 |
T.Nonaka,
A.Kita,
J.Miura-Ohnuma,
E.Katoh,
N.Inagaki,
T.Yamazaki,
and
K.Miki
(2005).
Crystal structure of putative N-acetyl-gamma-glutamyl-phosphate reductase (AK071544) from rice (Oryza sativa).
|
| |
Proteins,
61,
1137-1140.
|
 |
|
PDB code:
|
 |
|
|
|
|
|
 |
C.R.Faehnle,
J.Blanco,
and
R.E.Viola
(2004).
Structural basis for discrimination between oxyanion substrates or inhibitors in aspartate-beta-semialdehyde dehydrogenase.
|
| |
Acta Crystallogr D Biol Crystallogr,
60,
2320-2324.
|
 |
|
PDB codes:
|
 |
|
|
|
|
|
 |
J.Blanco,
R.A.Moore,
C.R.Faehnle,
D.M.Coe,
and
R.E.Viola
(2004).
The role of substrate-binding groups in the mechanism of aspartate-beta-semialdehyde dehydrogenase.
|
| |
Acta Crystallogr D Biol Crystallogr,
60,
1388-1395.
|
 |
|
PDB codes:
|
 |
|
|
|
|
|
 |
J.Blanco,
R.A.Moore,
C.R.Faehnle,
and
R.E.Viola
(2004).
Critical catalytic functional groups in the mechanism of aspartate-beta-semialdehyde dehydrogenase.
|
| |
Acta Crystallogr D Biol Crystallogr,
60,
1808-1815.
|
 |
|
 |
 |
|
The most recent references are shown first.
Citation data come partly from CiteXplore and partly
from an automated harvesting procedure. Note that this is likely to be
only a partial list as not all journals are covered by
either method. However, we are continually building up the citation data
so more and more references will be included with time.
Where a reference describes a PDB structure, the PDB
codes are
shown on the right.
|
 |

