 |
PDBsum entry 1m4l

|

|

|
|
 |
Contents |
 |
|
|

|

|
|
|
|
|
|
|
|
* Residue conservation analysis
|
|
|
|
 |
|
|
 |
 |
 |
 |
Enzyme class:
|
 |
E.C.3.4.17.1
- carboxypeptidase A.
|
|
 |
 |
 |
 |
 |
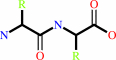
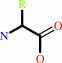
Reaction:
|
 |
Peptidyl-L-amino acid + H2O = peptide + L-amino acid
|
 |
 |
 |
 |
 |
|
+
|
|
=
|
|
+
|
|
|
 |
 |
 |
 |
 |
 |
 |
 |
Cofactor:
|
 |
Zn(2+)
|
 |
 |
 |
 |
 |
 |
 |
|
Molecule diagrams generated from .mol files obtained from the
KEGG ftp site
|
|
 |
 |
 |
 |
 |
 |
 |
 |
 |
 |
 |
 |
 |
 |
 |

|

|

|
| |
|
|
| |
|
DOI no:
|
Acta Crystallogr D Biol Crystallogr
59:323-333
(2003)
|
|
PubMed id:
|
|
|
|

|
| |
|
Refined structure of bovine carboxypeptidase A at 1.25 A resolution.
|
|
A.Kilshtain-Vardi,
M.Glick,
H.M.Greenblatt,
A.Goldblum,
G.Shoham.
|
|
|

|
| |
ABSTRACT
|
|

|
| |

|
The crystal structure of the bovine zinc metalloproteinase carboxypeptidase A
(CPA) has been refined to 1.25 A resolution based on room-temperature X-ray
synchrotron data. The significantly improved structure of CPA at this resolution
(anisotropic temperature factors, R factor = 10.4%, R(free) = 14.5%) allowed the
modelling of conformational disorders of side chains, improved the description
of the protein solvent network (375 water molecules) and provided a more
accurate picture of the interactions between the active-site zinc and its
ligands. The calculation of standard uncertainties in individual atom positions
of the refined model of CPA allowed the deduction of the protonation state of
some key residues in the active site and confirmed that Glu72 and Glu270 are
negatively charged in the resting state of the enzyme at pH 7.5. These results
were further validated by theoretical calculations that showed significant
reduction of the pK(a) of these side chains relative to solution values. The
distance between the zinc-bound solvent molecule and the metal ion is strongly
suggestive of a neutral water molecule and not a hydroxide ion in the resting
state of the enzyme. These findings could support both the general acid/general
base mechanism, as well as the anhydride mechanism suggested for CPA.

|

|
|
|

|
| |
Selected figure(s)
|
|

|
| |
 |
 |

|
 |

|
 |
Figure 1.
Figure 1 Electron-density `omit' map around the active site of
native CPA at 1.25 Å resolution. Electron-density contour
levels are at +4.5  (cyan).
Superimposed on the density is the final 1.25 Å model of CPA
(ball-and-stick representation, common atom colour codes),
featuring the zinc and its ligands, as well as the catalytic
residues Arg127 and Glu270. (cyan).
Superimposed on the density is the final 1.25 Å model of CPA
(ball-and-stick representation, common atom colour codes),
featuring the zinc and its ligands, as well as the catalytic
residues Arg127 and Glu270.
|
 |
Figure 5.
Figure 5 A stereoview of the active site of native CPA (stick
model) following the proton-addition algorithm. Atoms are
coloured according to conventional codes (C atoms, yellow; N
atoms, blue; O atoms, red; H atoms, white; Zn atoms, purple).
Zinc-ligand interactions are indicated (dashed lines) and their
distances are displayed (Å). The zinc-bound water is indicated
by `W'.
|
 |
|
|

|
| |
The above figures are
reprinted
by permission from the IUCr:
Acta Crystallogr D Biol Crystallogr
(2003,
59,
323-333)
copyright 2003.
|
|
| |
Figures were
selected
by an automated process.
|
|

|

|
|
 |
 |
|
 |
 |
 |
 |
 |
 |
 |
 |
 |
|
Literature references that cite this PDB file's key reference
|
|
 |
| |
PubMed id
|
 |
Reference
|
 |
|
|
|
 |
M.A.Zoroddu,
S.Medici,
M.Peana,
and
R.Anedda
(2010).
NMR studies of zinc binding in a multi-histidinic peptide fragment.
|
| |
Dalton Trans,
39,
1282-1294.
|
 |
|
|
|
|
 |
E.Bitto,
C.A.Bingman,
G.E.Wesenberg,
J.G.McCoy,
and
G.N.Phillips
(2007).
Structure of aspartoacylase, the brain enzyme impaired in Canavan disease.
|
| |
Proc Natl Acad Sci U S A,
104,
456-461.
|
 |
|
PDB codes:
|
 |
|
|
|
|
|
 |
A.Russ,
I.Pechik,
and
N.Andreeva
(2005).
Database for three dimensional structures of pepsin-like enzymes using the internal coordinate system.
|
| |
Proteins,
61,
223-226.
|
 |
|
|
|
|
 |
T.Suzuki,
K.Ishihara,
H.Migaki,
W.Matsuura,
A.Kohda,
K.Okumura,
M.Nagao,
Y.Yamaguchi-Iwai,
and
T.Kambe
(2005).
Zinc transporters, ZnT5 and ZnT7, are required for the activation of alkaline phosphatases, zinc-requiring enzymes that are glycosylphosphatidylinositol-anchored to the cytoplasmic membrane.
|
| |
J Biol Chem,
280,
637-643.
|
 |
|
|
|
|
 |
V.P.Denisov,
J.L.Schlessman,
B.García-Moreno E,
and
B.Halle
(2004).
Stabilization of internal charges in a protein: water penetration or conformational change?
|
| |
Biophys J,
87,
3982-3994.
|
 |
|
PDB code:
|
 |
|
|
 |
 |
|
The most recent references are shown first.
Citation data come partly from CiteXplore and partly
from an automated harvesting procedure. Note that this is likely to be
only a partial list as not all journals are covered by
either method. However, we are continually building up the citation data
so more and more references will be included with time.
Where a reference describes a PDB structure, the PDB
codes are
shown on the right.
|
 |

