 |
PDBsum entry 1k0e

|

|

|
|
 |
Contents |
 |
|
|

|

|
|
|
|
|
|
|
|
* Residue conservation analysis
|
|
|
 |
|
|
 |
 |
 |
 |
Enzyme class:
|
 |
Chains A, B:
E.C.2.6.1.85
- aminodeoxychorismate synthase.
|
|
 |
 |
 |
 |
 |
Reaction:
|
 |
chorismate + L-glutamine = 4-amino-4-deoxychorismate + L-glutamate
|
 |
 |
 |
 |
 |
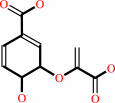 chorismate
chorismate
|
+
|
L-glutamine
Bound ligand (Het Group name = )
matches with 56.25% similarity
|
=
|
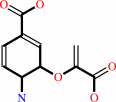 4-amino-4-deoxychorismate
4-amino-4-deoxychorismate
|
+
|
 L-glutamate
L-glutamate
|
|
 |
 |
 |
 |
 |
 |
 |
 |
Cofactor:
|
 |
Mg(2+)
|
 |
 |
 |
 |
 |
 |
 |
|
Molecule diagrams generated from .mol files obtained from the
KEGG ftp site
|
|
 |
 |
 |
 |
 |
 |
 |
 |
 |
 |
 |
 |
 |
 |
 |

|

|

|
| |
|
|
| |
|
DOI no:
|
Biochemistry
41:2198-2208
(2002)
|
|
PubMed id:
|
|
|
|

|
| |
|
Structure of Escherichia coli aminodeoxychorismate synthase: architectural conservation and diversity in chorismate-utilizing enzymes.
|
|
J.F.Parsons,
P.Y.Jensen,
A.S.Pachikara,
A.J.Howard,
E.Eisenstein,
J.E.Ladner.
|
|
|

|
| |
ABSTRACT
|
|

|
| |

|
Aminodeoxychorismate synthase is part of a heterodimeric complex that catalyzes
the two-step biosynthesis of 4-amino-4-deoxychorismate, a precursor of
p-aminobenzoate and folate in microorganisms. In the first step, a glutamine
amidotransferase encoded by the pabA gene generates ammonia as a substrate that,
along with chorismate, is used in the second step, catalyzed by
aminodeoxychorismate synthase, the product of the pabB gene. Here we report the
X-ray crystal structure of Escherichia coli PabB determined in two different
crystal forms, each at 2.0 A resolution. The 453-residue monomeric PabB has a
complex alpha/beta fold which is similar to that seen in the structures of
homologous, oligomeric TrpE subunits of several anthranilate synthases of
microbial origin. A comparison of the structures of these two classes of
chorismate-utilizing enzymes provides a rationale for the differences in
quaternary structures seen for these enzymes, and indicates that the weak or
transient association of PabB with PabA during catalysis stems at least partly
from a limited interface for protein interactions. Additional analyses of the
structures enabled the tentative identification of the active site of PabB,
which contains a number of residues implicated from previous biochemical and
genetic studies to be essential for activity. Differences in the structures
determined from phosphate- and formate-grown crystals, and the location of an
adventitious formate ion, suggest that conformational changes in loop regions
adjacent to the active site may be needed for catalysis. A surprising finding in
the structure of PabB was the presence of a tryptophan molecule deeply embedded
in a binding pocket that is analogous to the regulatory site in the TrpE
subunits of the anthranilate synthases. The strongly bound ligand, which cannot
be dissociated without denaturation of PabB, may play a structural role in the
enzyme since there is no effect of tryptophan on the enzymic synthesis of
aminodeoxychorismate. Extensive sequence similarity in the tryptophan-binding
pocket among several other chorismate-utilizing enzymes, including isochorismate
synthase, suggests that they too may bind tryptophan for structural integrity,
and corroborates early ideas on the evolution of this interesting enzyme family.

|

|
|
|

|

|
|
 |
 |
|
 |
 |
 |
 |
 |
 |
 |
 |
 |
|
Literature references that cite this PDB file's key reference
|
|
 |
| |
PubMed id
|
 |
Reference
|
 |
|
|
|
 |
S.M.Hashimi,
and
R.G.Birch
(2010).
Functional analysis of genes for benzoate metabolism in the albicidin biosynthetic region of Xanthomonas albilineans.
|
| |
Appl Microbiol Biotechnol,
87,
1475-1485.
|
 |
|
|
|
|
 |
R.J.Payne,
E.M.Bulloch,
M.M.Toscano,
M.A.Jones,
O.Kerbarh,
and
C.Abell
(2009).
Synthesis and evaluation of 2,5-dihydrochorismate analogues as inhibitors of the chorismate-utilising enzymes.
|
| |
Org Biomol Chem,
7,
2421-2429.
|
 |
|
|
|
|
 |
S.G.Van Lanen,
S.Lin,
and
B.Shen
(2008).
Biosynthesis of the enediyne antitumor antibiotic C-1027 involves a new branching point in chorismate metabolism.
|
| |
Proc Natl Acad Sci U S A,
105,
494-499.
|
 |
|
|
|
|
 |
A.Wegkamp,
W.van Oorschot,
W.M.de Vos,
and
E.J.Smid
(2007).
Characterization of the role of para-aminobenzoic acid biosynthesis in folate production by Lactococcus lactis.
|
| |
Appl Environ Microbiol,
73,
2673-2681.
|
 |
|
|
|
|
 |
D.E.Scott,
A.Ciulli,
and
C.Abell
(2007).
Coenzyme biosynthesis: enzyme mechanism, structure and inhibition.
|
| |
Nat Prod Rep,
24,
1009-1026.
|
 |
|
|
|
|
 |
A.J.Harrison,
M.Yu,
T.Gårdenborg,
M.Middleditch,
R.J.Ramsay,
E.N.Baker,
and
J.S.Lott
(2006).
The structure of MbtI from Mycobacterium tuberculosis, the first enzyme in the biosynthesis of the siderophore mycobactin, reveals it to be a salicylate synthase.
|
| |
J Bacteriol,
188,
6081-6091.
|
 |
|
PDB code:
|
 |
|
|
|
|
|
 |
E.M.Bulloch,
and
C.Abell
(2005).
Detection of covalent intermediates formed in the reaction of 4-amino-4-deoxychorismate synthase.
|
| |
Chembiochem,
6,
832-834.
|
 |
|
|
|
|
 |
R.J.Payne,
O.Kerbarh,
R.N.Miguel,
A.D.Abell,
and
C.Abell
(2005).
Inhibition studies on salicylate synthase.
|
| |
Org Biomol Chem,
3,
1825-1827.
|
 |
|
|
|
|
 |
M.Goto,
R.Omi,
J.Hoseki,
N.Nakagawa,
I.Miyahara,
and
K.Hirotsu
(2003).
Expression, purification and preliminary X-ray characterization of CTP synthetase from Thermus thermophilus HB8.
|
| |
Acta Crystallogr D Biol Crystallogr,
59,
551-553.
|
 |
|
 |
 |
|
The most recent references are shown first.
Citation data come partly from CiteXplore and partly
from an automated harvesting procedure. Note that this is likely to be
only a partial list as not all journals are covered by
either method. However, we are continually building up the citation data
so more and more references will be included with time.
Where a reference describes a PDB structure, the PDB
code is
shown on the right.
|
 |
|

