 |
PDBsum entry 1htt

|
|
|

|
 |

|

|

|

|

|

|

|

|

|

|

|

|

|

|

|

|

|

|
 |

|

|

|
 |
 |
 |
 |
 |
 |
 |
 |
 |
 |
 |
 |
 |
 |
 |
 |
 |
 |
 |
 |

|

|

|

|

|

|

|

|

|
|
Complex (tRNA synthetase/his-adenylate)
|
PDB id
|
|

|

|
1htt
|

|

|

|

|

|

|

|

|

|

|

|

|

|

|

|

|

|

|

|
|
 |
Contents |
 |
|
|

|

|
|
|
|
|
|
|
|
* Residue conservation analysis
|
|
|

|
|
PDB id:
|
 |
|
 |
| Name: |
 |
Complex (tRNA synthetase/his-adenylate)
|
 |
|
Title:
|
 |
Histidyl-tRNA synthetase
|
|
Structure:
|
 |
Histidyl-tRNA synthetase. Chain: a, b, c, d. Synonym: histidine-tRNA ligase. Engineered: yes
|
|
Source:
|
 |
Escherichia coli. Organism_taxid: 562. Strain: jm109. Expressed in: escherichia coli. Expression_system_taxid: 562.
|
|
Biol. unit:
|
 |
Tetramer (from
)
|
|
Resolution:
|
 |
|
2.60Å
|
R-factor:
|
0.246
|
R-free:
|
0.327
|
|
|
Authors:
|
 |
J.G.Arnez,D.C.Harris,A.Mitschler,B.Rees,C.S.Francklyn,D.Moras
|
|
Key ref:
|
 |
J.G.Arnez
et al.
(1995).
Crystal structure of histidyl-tRNA synthetase from Escherichia coli complexed with histidyl-adenylate.
Embo J,
14,
4143-4155.
PubMed id:
|
 |
|
Date:
|
 |
|
09-Mar-96
|
Release date:
|
27-Jan-97
|
|
|

|

|
|
PROCHECK
|

|

|
|
|
Headers
|
 |
|
|
References
|
|
|
|

|

|
|
P60906
(SYH_ECOLI) -
Histidine--tRNA ligase from Escherichia coli (strain K12)
|
|

|

|
Seq:
Struc:
|
 |
 |
 |
424 a.a.
366 a.a.
|

|

|

|

|
|

|

|

|
 |
 |

|
|
Key: |
 |
PfamA domain |
 |
 |
 |
Secondary structure |
 |
 |
CATH domain |
 |
|

|
|
|
 |
|
|
 |
 |
 |
 |
Enzyme class:
|
 |
E.C.6.1.1.21
- histidine--tRNA ligase.
|
|
 |
 |
 |
 |
 |
Reaction:
|
 |
tRNA(His) + L-histidine + ATP = L-histidyl-tRNA(His) + AMP + diphosphate + H+
|
 |
 |
 |
 |
 |
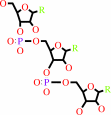 tRNA(His)
tRNA(His)
|
+
|
L-histidine
Bound ligand (Het Group name = )
matches with 90.91% similarity
|
+
|
 ATP
ATP
|
=
|
L-histidyl-tRNA(His)
Bound ligand (Het Group name = )
corresponds exactly
|
+
|
 AMP
AMP
|
+
|
 diphosphate
diphosphate
|
+
|
H(+)
|
|
 |
 |
 |
 |
 |
 |
 |
 |
 |
 |
|
Molecule diagrams generated from .mol files obtained from the
KEGG ftp site
|
|
 |
 |
 |
 |
 |
 |
 |
 |
 |
 |
 |
 |
 |
 |
 |

|

|

|
| |
|
|
| |
|
|
Embo J
14:4143-4155
(1995)
|
|
PubMed id:
|
|
|
|

|
| |
|
Crystal structure of histidyl-tRNA synthetase from Escherichia coli complexed with histidyl-adenylate.
|
|
J.G.Arnez,
D.C.Harris,
A.Mitschler,
B.Rees,
C.S.Francklyn,
D.Moras.
|
|
|

|
| |
ABSTRACT
|
|

|
| |

|
The crystal structure at 2.6 A of the histidyl-tRNA synthetase from Escherichia
coli complexed with histidyl-adenylate has been determined. The enzyme is a
homodimer with a molecular weight of 94 kDa and belongs to the class II of
aminoacyl-tRNA synthetases (aaRS). The asymmetric unit is composed of two
homodimers. Each monomer consists of two domains. The N-terminal catalytic core
domain contains a six-stranded antiparallel beta-sheet sitting on two
alpha-helices, which can be superposed with the catalytic domains of yeast
AspRS, and GlyRS and SerRS from Thermus thermophilus with a root-mean-square
difference on the C alpha atoms of 1.7-1.9 A. The active sites of all four
monomers are occupied by histidyl-adenylate, which apparently forms during
crystallization. The 100 residue C-terminal alpha/beta domain resembles half of
a beta-barrel, and provides an independent domain oriented to contact the
anticodon stem and part of the anticodon loop of tRNA(His). The modular domain
organization of histidyl-tRNA synthetase reiterates a repeated theme in aaRS,
and its structure should provide insight into the ability of certain aaRS to
aminoacylate minihelices and other non-tRNA molecules.

|

|
|
|

|

|
|
 |
 |
|
 |
 |
 |
 |
 |
 |
 |
 |
 |
|
Literature references that cite this PDB file's key reference
|
|
 |
| |
PubMed id
|
 |
Reference
|
 |
|
|
|
 |
S.B.Pierce,
K.M.Chisholm,
E.D.Lynch,
M.K.Lee,
T.Walsh,
J.M.Opitz,
W.Li,
R.E.Klevit,
and
M.C.King
(2011).
Mutations in mitochondrial histidyl tRNA synthetase HARS2 cause ovarian dysgenesis and sensorineural hearing loss of Perrault syndrome.
|
| |
Proc Natl Acad Sci U S A,
108,
6543-6548.
|
 |
|
|
|
|
 |
E.A.Merritt,
T.L.Arakaki,
J.R.Gillespie,
E.T.Larson,
A.Kelley,
N.Mueller,
A.J.Napuli,
J.Kim,
L.Zhang,
C.L.Verlinde,
E.Fan,
F.Zucker,
F.S.Buckner,
W.C.van Voorhis,
and
W.G.Hol
(2010).
Crystal structures of trypanosomal histidyl-tRNA synthetase illuminate differences between eukaryotic and prokaryotic homologs.
|
| |
J Mol Biol,
397,
481-494.
|
 |
|
PDB codes:
|
 |
|
|
|
|
|
 |
S.Shaul,
D.Berel,
Y.Benjamini,
and
D.Graur
(2010).
Revisiting the operational RNA code for amino acids: Ensemble attributes and their implications.
|
| |
RNA,
16,
141-153.
|
 |
|
|
|
|
 |
B.Kimura,
H.Takahashi,
S.Hokimoto,
Y.Tanaka,
and
T.Fujii
(2009).
Induction of the histidine decarboxylase genes of Photobacterium damselae subsp. damselae (formally P. histaminum) at low pH.
|
| |
J Appl Microbiol,
107,
485-497.
|
 |
|
|
|
|
 |
E.Guth,
M.Farris,
M.Bovee,
and
C.S.Francklyn
(2009).
Asymmetric amino acid activation by class II histidyl-tRNA synthetase from Escherichia coli.
|
| |
J Biol Chem,
284,
20753-20762.
|
 |
|
|
|
|
 |
G.Sharma,
and
E.A.First
(2009).
Thermodynamic Analysis Reveals a Temperature-dependent Change in the Catalytic Mechanism of Bacillus stearothermophilus Tyrosyl-tRNA Synthetase.
|
| |
J Biol Chem,
284,
4179-4190.
|
 |
|
|
|
|
 |
S.M.Levine,
N.Raben,
D.Xie,
F.B.Askin,
R.Tuder,
M.Mullins,
A.Rosen,
and
L.A.Casciola-Rosen
(2007).
Novel conformation of histidyl-transfer RNA synthetase in the lung: the target tissue in Jo-1 autoantibody-associated myositis.
|
| |
Arthritis Rheum,
56,
2729-2739.
|
 |
|
|
|
|
 |
A.G.Hinnebusch
(2005).
Translational regulation of GCN4 and the general amino acid control of yeast.
|
| |
Annu Rev Microbiol,
59,
407-450.
|
 |
|
|
|
|
 |
A.K.Padyana,
H.Qiu,
A.Roll-Mecak,
A.G.Hinnebusch,
and
S.K.Burley
(2005).
Structural basis for autoinhibition and mutational activation of eukaryotic initiation factor 2alpha protein kinase GCN2.
|
| |
J Biol Chem,
280,
29289-29299.
|
 |
|
PDB codes:
|
 |
|
|
|
|
|
 |
K.S.Champagne,
M.Sissler,
Y.Larrabee,
S.Doublié,
and
C.S.Francklyn
(2005).
Activation of the hetero-octameric ATP phosphoribosyl transferase through subunit interface rearrangement by a tRNA synthetase paralog.
|
| |
J Biol Chem,
280,
34096-34104.
|
 |
|
PDB codes:
|
 |
|
|
|
|
|
 |
M.L.Bovee,
M.A.Pierce,
and
C.S.Francklyn
(2003).
Induced fit and kinetic mechanism of adenylation catalyzed by Escherichia coli threonyl-tRNA synthetase.
|
| |
Biochemistry,
42,
15102-15113.
|
 |
|
|
|
|
 |
M.Seetharaman,
C.Williams,
C.J.Cramer,
and
K.Musier-Forsyth
(2003).
Effect of G-1 on histidine tRNA microhelix conformation.
|
| |
Nucleic Acids Res,
31,
7311-7321.
|
 |
|
|
|
|
 |
H.Qiu,
J.Dong,
C.Hu,
C.S.Francklyn,
and
A.G.Hinnebusch
(2001).
The tRNA-binding moiety in GCN2 contains a dimerization domain that interacts with the kinase domain and is required for tRNA binding and kinase activation.
|
| |
EMBO J,
20,
1425-1438.
|
 |
|
|
|
|
 |
R.Fishman,
V.Ankilova,
N.Moor,
and
M.Safro
(2001).
Structure at 2.6 A resolution of phenylalanyl-tRNA synthetase complexed with phenylalanyl-adenylate in the presence of manganese.
|
| |
Acta Crystallogr D Biol Crystallogr,
57,
1534-1544.
|
 |
|
PDB code:
|
 |
|
|
|
|
|
 |
S.A.Hawko,
and
C.S.Francklyn
(2001).
Covariation of a specificity-determining structural motif in an aminoacyl-tRNA synthetase and a tRNA identity element.
|
| |
Biochemistry,
40,
1930-1936.
|
 |
|
|
|
|
 |
A.K.Forrest,
R.L.Jarvest,
L.M.Mensah,
P.J.O'Hanlon,
A.J.Pope,
and
R.J.Sheppard
(2000).
Aminoalkyl adenylate and aminoacyl sulfamate intermediate analogues differing greatly in affinity for their cognate Staphylococcus aureus aminoacyl tRNA synthetases.
|
| |
Bioorg Med Chem Lett,
10,
1871-1874.
|
 |
|
|
|
|
 |
A.Yaremchuk,
S.Cusack,
and
M.Tukalo
(2000).
Crystal structure of a eukaryote/archaeon-like protyl-tRNA synthetase and its complex with tRNAPro(CGG).
|
| |
EMBO J,
19,
4745-4758.
|
 |
|
|
|
|
 |
I.Sugiura,
O.Nureki,
Y.Ugaji-Yoshikawa,
S.Kuwabara,
A.Shimada,
M.Tateno,
B.Lorber,
R.Giegé,
D.Moras,
S.Yokoyama,
and
M.Konno
(2000).
The 2.0 A crystal structure of Thermus thermophilus methionyl-tRNA synthetase reveals two RNA-binding modules.
|
| |
Structure,
8,
197-208.
|
 |
|
PDB code:
|
 |
|
|
|
|
|
 |
J.Dong,
H.Qiu,
M.Garcia-Barrio,
J.Anderson,
and
A.G.Hinnebusch
(2000).
Uncharged tRNA activates GCN2 by displacing the protein kinase moiety from a bipartite tRNA-binding domain.
|
| |
Mol Cell,
6,
269-279.
|
 |
|
|
|
|
 |
K.A.Denessiouk,
and
M.S.Johnson
(2000).
When fold is not important: a common structural framework for adenine and AMP binding in 12 unrelated protein families.
|
| |
Proteins,
38,
310-326.
|
 |
|
|
|
|
 |
M.Ibba,
and
D.Soll
(2000).
Aminoacyl-tRNA synthesis.
|
| |
Annu Rev Biochem,
69,
617-650.
|
 |
|
|
|
|
 |
M.Kaminska,
M.Deniziak,
P.Kerjan,
J.Barciszewski,
and
M.Mirande
(2000).
A recurrent general RNA binding domain appended to plant methionyl-tRNA synthetase acts as a cis-acting cofactor for aminoacylation.
|
| |
EMBO J,
19,
6908-6917.
|
 |
|
|
|
|
 |
R.Sood,
A.C.Porter,
D.A.Olsen,
D.R.Cavener,
and
R.C.Wek
(2000).
A mammalian homologue of GCN2 protein kinase important for translational control by phosphorylation of eukaryotic initiation factor-2alpha.
|
| |
Genetics,
154,
787-801.
|
 |
|
|
|
|
 |
V.Cura,
D.Moras,
and
D.Kern
(2000).
Sequence analysis and modular organization of threonyl-tRNA synthetase from Thermus thermophilus and its interrelation with threonyl-tRNA synthetases of other origins.
|
| |
Eur J Biochem,
267,
379-393.
|
 |
|
|
|
|
 |
V.Guez,
S.Nair,
A.Chaffotte,
and
H.Bedouelle
(2000).
The anticodon-binding domain of tyrosyl-tRNA synthetase: state of folding and origin of the crystallographic disorder.
|
| |
Biochemistry,
39,
1739-1747.
|
 |
|
|
|
|
 |
A.J.Morales,
M.A.Swairjo,
and
P.Schimmel
(1999).
Structure-specific tRNA-binding protein from the extreme thermophile Aquifex aeolicus.
|
| |
EMBO J,
18,
3475-3483.
|
 |
|
|
|
|
 |
M.Sissler,
C.Delorme,
J.Bond,
S.D.Ehrlich,
P.Renault,
and
C.Francklyn
(1999).
An aminoacyl-tRNA synthetase paralog with a catalytic role in histidine biosynthesis.
|
| |
Proc Natl Acad Sci U S A,
96,
8985-8990.
|
 |
|
|
|
|
 |
W.Freist,
J.F.Verhey,
A.Rühlmann,
D.H.Gauss,
and
J.G.Arnez
(1999).
Histidyl-tRNA synthetase.
|
| |
Biol Chem,
380,
623-646.
|
 |
|
|
|
|
 |
B.Felden,
and
R.Giegé
(1998).
Resected RNA pseudoknots and their recognition by histidyl-tRNA synthetase.
|
| |
Proc Natl Acad Sci U S A,
95,
10431-10436.
|
 |
|
|
|
|
 |
C.Briand,
A.Poterszman,
A.Mitschler,
M.Yusupov,
J.C.Thierry,
and
D.Moras
(1998).
Crystals of Thermus thermophilus tRNAAsp complexed with its cognate aspartyl-tRNA synthetase have a solvent content of 75%. Comparison with other aminoacylation systems.
|
| |
Acta Crystallogr D Biol Crystallogr,
54,
1382-1386.
|
 |
|
|
|
|
 |
D.S.Olsen,
B.Jordan,
D.Chen,
R.C.Wek,
and
D.R.Cavener
(1998).
Isolation of the gene encoding the Drosophila melanogaster homolog of the Saccharomyces cerevisiae GCN2 eIF-2alpha kinase.
|
| |
Genetics,
149,
1495-1509.
|
 |
|
|
|
|
 |
E.Sattlegger,
A.G.Hinnebusch,
and
I.B.Barthelmess
(1998).
cpc-3, the Neurospora crassa homologue of yeast GCN2, encodes a polypeptide with juxtaposed eIF2alpha kinase and histidyl-tRNA synthetase-related domains required for general amino acid control.
|
| |
J Biol Chem,
273,
20404-20416.
|
 |
|
|
|
|
 |
J.Cavarelli,
B.Delagoutte,
G.Eriani,
J.Gangloff,
and
D.Moras
(1998).
L-arginine recognition by yeast arginyl-tRNA synthetase.
|
| |
EMBO J,
17,
5438-5448.
|
 |
|
PDB code:
|
 |
|
|
|
|
|
 |
L.Ribas de Pouplana,
D.Buechter,
N.Y.Sardesai,
and
P.Schimmel
(1998).
Functional analysis of peptide motif for RNA microhelix binding suggests new family of RNA-binding domains.
|
| |
EMBO J,
17,
5449-5457.
|
 |
|
|
|
|
 |
R.Brouwer,
W.Vree Egberts,
P.H.Jongen,
B.G.van Engelen,
and
W.J.van Venrooij
(1998).
Frequent occurrence of anti-tRNA(His) autoantibodies that recognize a conformational epitope in sera of patients with myositis.
|
| |
Arthritis Rheum,
41,
1428-1437.
|
 |
|
|
|
|
 |
S.Cusack,
A.Yaremchuk,
I.Krikliviy,
and
M.Tukalo
(1998).
tRNA(Pro) anticodon recognition by Thermus thermophilus prolyl-tRNA synthetase.
|
| |
Structure,
6,
101-108.
|
 |
|
|
|
|
 |
T.Nakatsu,
H.Kato,
and
J.Oda
(1998).
Crystal structure of asparagine synthetase reveals a close evolutionary relationship to class II aminoacyl-tRNA synthetase.
|
| |
Nat Struct Biol,
5,
15-19.
|
 |
|
PDB codes:
|
 |
|
|
|
|
|
 |
V.L.Rath,
L.F.Silvian,
B.Beijer,
B.S.Sproat,
and
T.A.Steitz
(1998).
How glutaminyl-tRNA synthetase selects glutamine.
|
| |
Structure,
6,
439-449.
|
 |
|
PDB code:
|
 |
|
|
|
|
|
 |
A.Aberg,
A.Yaremchuk,
M.Tukalo,
B.Rasmussen,
and
S.Cusack
(1997).
Crystal structure analysis of the activation of histidine by Thermus thermophilus histidyl-tRNA synthetase.
|
| |
Biochemistry,
36,
3084-3094.
|
 |
|
PDB codes:
|
 |
|
|
|
|
|
 |
B.F.Clark,
and
J.Nyborg
(1997).
The ternary complex of EF-Tu and its role in protein biosynthesis.
|
| |
Curr Opin Struct Biol,
7,
110-116.
|
 |
|
|
|
|
 |
C.Stehlin,
D.H.Heacock,
H.Liu,
and
K.Musier-Forsyth
(1997).
Chemical modification and site-directed mutagenesis of the single cysteine in motif 3 of class II Escherichia coli prolyl-tRNA synthetase.
|
| |
Biochemistry,
36,
2932-2938.
|
 |
|
|
|
|
 |
J.Augustine,
and
C.Francklyn
(1997).
Design of an active fragment of a class II aminoacyl-tRNA synthetase and its significance for synthetase evolution.
|
| |
Biochemistry,
36,
3473-3482.
|
 |
|
|
|
|
 |
J.G.Arnez,
J.G.Augustine,
D.Moras,
and
C.S.Francklyn
(1997).
The first step of aminoacylation at the atomic level in histidyl-tRNA synthetase.
|
| |
Proc Natl Acad Sci U S A,
94,
7144-7149.
|
 |
|
PDB codes:
|
 |
|
|
|
|
|
 |
J.Rudinger,
B.Felden,
C.Florentz,
and
R.Giegé
(1997).
Strategy for RNA recognition by yeast histidyl-tRNA synthetase.
|
| |
Bioorg Med Chem,
5,
1001-1009.
|
 |
|
|
|
|
 |
S.Cusack
(1997).
Aminoacyl-tRNA synthetases.
|
| |
Curr Opin Struct Biol,
7,
881-889.
|
 |
|
|
|
|
 |
F.Agou,
J.P.Waller,
and
M.Mirande
(1996).
Expression of rat aspartyl-tRNA synthetase in Saccharomyces cerevisiae. Role of the NH2-terminal polypeptide extension on enzyme activity and stability.
|
| |
J Biol Chem,
271,
29295-29303.
|
 |
|
|
|
|
 |
P.Romby,
J.Caillet,
C.Ebel,
C.Sacerdot,
M.Graffe,
F.Eyermann,
C.Brunel,
H.Moine,
C.Ehresmann,
B.Ehresmann,
and
M.Springer
(1996).
The expression of E.coli threonyl-tRNA synthetase is regulated at the translational level by symmetrical operator-repressor interactions.
|
| |
EMBO J,
15,
5976-5987.
|
 |
|
|
|
|
 |
R.K.Airas
(1996).
Differences in the magnesium dependences of the class I and class II aminoacyl-tRNA synthetases from Escherichia coli.
|
| |
Eur J Biochem,
240,
223-231.
|
 |
|
|
|
|
 |
S.Brenner,
and
L.M.Corrochano
(1996).
Translocation events in the evolution of aminoacyl-tRNA synthetases.
|
| |
Proc Natl Acad Sci U S A,
93,
8485-8489.
|
 |
|
|
|
|
 |
S.Gillet,
C.B.Hoang,
J.M.Schmitter,
T.Fukui,
S.Blanquet,
and
C.Hountondji
(1996).
Affinity labeling of Escherichia coli histidyl-tRNA synthetase with reactive ATP analogues. Identification of labeled amino acid residues by matrix assisted laser desorption-ionization mass spectrometry.
|
| |
Eur J Biochem,
241,
133-141.
|
 |
|
|
|
|
 |
S.P.Hale,
and
P.Schimmel
(1996).
Protein synthesis editing by a DNA aptamer.
|
| |
Proc Natl Acad Sci U S A,
93,
2755-2758.
|
 |
|
|
|
|
 |
S.Zhu,
A.Y.Sobolev,
and
R.C.Wek
(1996).
Histidyl-tRNA synthetase-related sequences in GCN2 protein kinase regulate in vitro phosphorylation of eIF-2.
|
| |
J Biol Chem,
271,
24989-24994.
|
 |
|
|
|
|
 |
D.T.Logan,
M.H.Mazauric,
D.Kern,
and
D.Moras
(1995).
Crystal structure of glycyl-tRNA synthetase from Thermus thermophilus.
|
| |
EMBO J,
14,
4156-4167.
|
 |
|
PDB code:
|
 |
|
|
 |
 |
|
The most recent references are shown first.
Citation data come partly from CiteXplore and partly
from an automated harvesting procedure. Note that this is likely to be
only a partial list as not all journals are covered by
either method. However, we are continually building up the citation data
so more and more references will be included with time.
Where a reference describes a PDB structure, the PDB
codes are
shown on the right.
|
 |

