 |
PDBsum entry 1gof

|
|
|

|
 |

|

|

|

|

|

|

|

|

|

|

|

|

|

|

|

|
 |

|

|

|
 |
 |
 |
 |
 |
 |
 |
 |
 |
 |
 |
 |
 |
 |
 |
 |
 |
 |
 |

|

|

|

|

|

|

|

|

|
|
Oxidoreductase(oxygen(a))
|
PDB id
|
|

|

|
1gof
|

|

|

|

|

|

|

|

|

|

|

|

|

|

|

|

|

|

|

|
|
 |
Contents |
 |
|
|

|

|
|
|
|
|
|
|
|
|
|
* Residue conservation analysis
|
|
|
|
 |
|
|
 |
 |
 |
 |
Enzyme class:
|
 |
E.C.1.1.3.9
- galactose oxidase.
|
|
 |
 |
 |
 |
 |
Reaction:
|
 |
D-galactose + O2 = D-galacto-hexodialdose + H2O2
|
 |
 |
 |
 |
 |
 D-galactose
D-galactose
|
+
|
 O2
O2
|
=
|
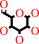 D-galacto-hexodialdose
D-galacto-hexodialdose
|
+
|
 H2O2
H2O2
|
|
 |
 |
 |
 |
 |
 |
 |
 |
Cofactor:
|
 |
Cu cation
|
 |
 |
 |
 |
 |
 |
 |
|
Molecule diagrams generated from .mol files obtained from the
KEGG ftp site
|
|
 |
 |
 |
 |
 |
 |
 |
 |
 |
 |
 |
 |
 |
 |
 |

|

|

|
| |
|
|
| |
|
|
Nature
350:87-90
(1991)
|
|
PubMed id:
|
|
|
|

|
| |
|
Novel thioether bond revealed by a 1.7 A crystal structure of galactose oxidase.
|
|
N.Ito,
S.E.Phillips,
C.Stevens,
Z.B.Ogel,
M.J.McPherson,
J.N.Keen,
K.D.Yadav,
P.F.Knowles.
|
|
|

|
| |
ABSTRACT
|
|

|
| |

|
Galactose oxidase is an extracellular enzyme secreted by the fungus Dactylium
dendroides. It is monomeric, with a relative molecular mass of 68,000, catalyses
the stereospecific oxidation of a broad range of primary alcohol substrates and
possesses a unique mononuclear copper site essential for catalysing a
two-electron transfer reaction during the oxidation of primary alcohols to
corresponding aldehydes. Recent evidence arguing against a Cu(III)-Cu(I) couple
implies the existence of a second redox-active site proposed to involve
pyrroloquinoline quinone or a tyrosine radical. We now report the crystal
structure of galactose oxidase at 1.7 A resolution. This reveals a unique
structural feature at the copper site with a novel thioether bond linking Cys
228 and Tyr 272 in a stacking interaction with Trp 290. We propose that these
molecular components stabilize the protein free-radical species essential for
catalysis and thus provide a 'built-in' secondary cofactor. This feature may
represent a new mechanism for mediating electron transfer in metalloenzymes in
the absence of exogenous cofactors.

|

|
|
|

|

|
|
 |
 |
|
 |
 |
 |
 |
 |
 |
 |
 |
 |
|
Literature references that cite this PDB file's key reference
|
|
 |
| |
PubMed id
|
 |
Reference
|
 |
|
|
|
 |
A.Mijovilovich,
S.Hamman,
F.Thomas,
F.M.de Groot,
and
B.M.Weckhuysen
(2011).
Protonation of the oxygen axial ligand in galactose oxidase model compounds as seen with high resolution X-ray emission experiments and FEFF simulations.
|
| |
Phys Chem Chem Phys,
13,
5600-5604.
|
 |
|
|
|
|
 |
H.J.Kang,
and
E.N.Baker
(2011).
Intramolecular isopeptide bonds: protein crosslinks built for stress?
|
| |
Trends Biochem Sci,
36,
229-237.
|
 |
|
|
|
|
 |
M.H.Stipanuk,
C.R.Simmons,
P.Andrew Karplus,
and
J.E.Dominy
(2011).
Thiol dioxygenases: unique families of cupin proteins.
|
| |
Amino Acids,
41,
91.
|
 |
|
|
|
|
 |
V.L.Davidson
(2011).
Generation of protein-derived redox cofactors by posttranslational modification.
|
| |
Mol Biosyst,
7,
29-37.
|
 |
|
|
|
|
 |
F.Aparecido Cordeiro,
C.Bertechini Faria,
and
I.Parra Barbosa-Tessmann
(2010).
Identification of new galactose oxidase genes in Fusarium spp.
|
| |
J Basic Microbiol,
50,
527-537.
|
 |
|
|
|
|
 |
H.Arora,
C.Philouze,
O.Jarjayes,
and
F.Thomas
(2010).
Co(II), Ni(II), Cu(II) and Zn(II) complexes of a bipyridine bis-phenol conjugate: generation and properties of coordinated radical species.
|
| |
Dalton Trans,
39,
10088-10098.
|
 |
|
|
|
|
 |
J.A.Worrall,
and
E.Vijgenboom
(2010).
Copper mining in Streptomyces: enzymes, natural products and development.
|
| |
Nat Prod Rep,
27,
742-756.
|
 |
|
|
|
|
 |
O.Spadiut,
L.Olsson,
and
H.Brumer
(2010).
A comparative summary of expression systems for the recombinant production of galactose oxidase.
|
| |
Microb Cell Fact,
9,
68.
|
 |
|
|
|
|
 |
S.Kalkhof,
S.Haehn,
M.Paulsson,
N.Smyth,
J.Meiler,
and
A.Sinz
(2010).
Computational modeling of laminin N-terminal domains using sparse distance constraints from disulfide bonds and chemical cross-linking.
|
| |
Proteins,
78,
3409-3427.
|
 |
|
|
|
|
 |
S.M.Lippow,
T.S.Moon,
S.Basu,
S.H.Yoon,
X.Li,
B.A.Chapman,
K.Robison,
D.Lipovšek,
and
K.L.Prather
(2010).
Engineering enzyme specificity using computational design of a defined-sequence library.
|
| |
Chem Biol,
17,
1306-1315.
|
 |
|
|
|
|
 |
F.Michel,
S.Hamman,
C.Philouze,
C.P.Del Valle,
E.Saint-Aman,
and
F.Thomas
(2009).
Galactose oxidase models: insights from 19F NMR spectroscopy.
|
| |
Dalton Trans,
(),
832-842.
|
 |
|
|
|
|
 |
K.J.Humphreys,
L.M.Mirica,
Y.Wang,
and
J.P.Klinman
(2009).
Galactose oxidase as a model for reactivity at a copper superoxide center.
|
| |
J Am Chem Soc,
131,
4657-4663.
|
 |
|
|
|
|
 |
S.K.Alamsetti,
S.Mannam,
P.Mutupandi,
and
G.Sekar
(2009).
Galactose oxidase model: biomimetic enantiomer-differentiating oxidation of alcohols by a chiral copper complex.
|
| |
Chemistry,
15,
1086-1090.
|
 |
|
|
|
|
 |
Y.Shimazaki,
M.Takani,
and
O.Yamauchi
(2009).
Metal complexes of amino acids and amino acid side chain groups. Structures and properties.
|
| |
Dalton Trans,
(),
7854-7869.
|
 |
|
|
|
|
 |
A.John,
M.M.Shaikh,
and
P.Ghosh
(2008).
Structural and functional mimic of galactose oxidase by a copper complex of a sterically demanding [N2O2] ligand.
|
| |
Dalton Trans,
(),
2815-2824.
|
 |
|
|
|
|
 |
D.Rokhsana,
D.M.Dooley,
and
R.K.Szilagyi
(2008).
Systematic development of computational models for the catalytic site in galactose oxidase: impact of outer-sphere residues on the geometric and electronic structures.
|
| |
J Biol Inorg Chem,
13,
371-383.
|
 |
|
|
|
|
 |
E.Severi,
A.Müller,
J.R.Potts,
A.Leech,
D.Williamson,
K.S.Wilson,
and
G.H.Thomas
(2008).
Sialic acid mutarotation is catalyzed by the Escherichia coli beta-propeller protein YjhT.
|
| |
J Biol Chem,
283,
4841-4849.
|
 |
|
PDB code:
|
 |
|
|
|
|
|
 |
F.Escalettes,
and
N.J.Turner
(2008).
Directed evolution of galactose oxidase: generation of enantioselective secondary alcohol oxidases.
|
| |
Chembiochem,
9,
857-860.
|
 |
|
|
|
|
 |
J.E.Dominy,
J.Hwang,
S.Guo,
L.L.Hirschberger,
S.Zhang,
and
M.H.Stipanuk
(2008).
Synthesis of amino acid cofactor in cysteine dioxygenase is regulated by substrate and represents a novel post-translational regulation of activity.
|
| |
J Biol Chem,
283,
12188-12201.
|
 |
|
|
|
|
 |
K.S.Aragão,
M.Satre,
A.Imberty,
and
A.Varrot
(2008).
Structure determination of Discoidin II from Dictyostelium discoideum and carbohydrate binding properties of the lectin domain.
|
| |
Proteins,
73,
43-52.
|
 |
|
PDB codes:
|
 |
|
|
|
|
|
 |
L.Que,
and
W.B.Tolman
(2008).
Biologically inspired oxidation catalysis.
|
| |
Nature,
455,
333-340.
|
 |
|
|
|
|
 |
M.S.Rogers,
R.Hurtado-Guerrero,
S.J.Firbank,
M.A.Halcrow,
D.M.Dooley,
S.E.Phillips,
P.F.Knowles,
and
M.J.McPherson
(2008).
Cross-link formation of the cysteine 228-tyrosine 272 catalytic cofactor of galactose oxidase does not require dioxygen.
|
| |
Biochemistry,
47,
10428-10439.
|
 |
|
PDB codes:
|
 |
|
|
|
|
|
 |
M.Stylianou,
C.Drouza,
Z.Viskadourakis,
J.Giapintzakis,
and
A.D.Keramidas
(2008).
Synthesis, structure, magnetic properties and aqueous solution characterization of p-hydroquinone and phenol iminodiacetate copper(II) complexes.
|
| |
Dalton Trans,
(),
6188-6204.
|
 |
|
|
|
|
 |
T.J.Stevens,
and
M.Paoli
(2008).
RCC1-like repeat proteins: a pangenomic, structurally diverse new superfamily of beta-propeller domains.
|
| |
Proteins,
70,
378-387.
|
 |
|
|
|
|
 |
Y.K.Lee,
M.M.Whittaker,
and
J.W.Whittaker
(2008).
The electronic structure of the Cys-Tyr(*) free radical in galactose oxidase determined by EPR spectroscopy.
|
| |
Biochemistry,
47,
6637-6649.
|
 |
|
|
|
|
 |
G.N.Phillips,
B.G.Fox,
J.L.Markley,
B.F.Volkman,
E.Bae,
E.Bitto,
C.A.Bingman,
R.O.Frederick,
J.G.McCoy,
B.L.Lytle,
B.S.Pierce,
J.Song,
and
S.N.Twigger
(2007).
Structures of proteins of biomedical interest from the Center for Eukaryotic Structural Genomics.
|
| |
J Struct Funct Genomics,
8,
73-84.
|
 |
|
|
|
|
 |
M.S.Rogers,
E.M.Tyler,
N.Akyumani,
C.R.Kurtis,
R.K.Spooner,
S.E.Deacon,
S.Tamber,
S.J.Firbank,
K.Mahmoud,
P.F.Knowles,
S.E.Phillips,
M.J.McPherson,
and
D.M.Dooley
(2007).
The stacking tryptophan of galactose oxidase: a second-coordination sphere residue that has profound effects on tyrosyl radical behavior and enzyme catalysis.
|
| |
Biochemistry,
46,
4606-4618.
|
 |
|
PDB codes:
|
 |
|
|
|
|
|
 |
N.Pletneva,
V.Pletnev,
T.Tikhonova,
A.A.Pakhomov,
V.Popov,
V.I.Martynov,
A.Wlodawer,
Z.Dauter,
and
S.Pletnev
(2007).
Refined crystal structures of red and green fluorescent proteins from the button polyp Zoanthus.
|
| |
Acta Crystallogr D Biol Crystallogr,
63,
1082-1093.
|
 |
|
PDB codes:
|
 |
|
|
|
|
|
 |
O.Rotthaus,
O.Jarjayes,
F.Thomas,
C.Philouze,
E.Saint-Aman,
and
J.L.Pierre
(2007).
Up to four phenoxyl radicals coordinated to two metal ions in copper and zinc complexes?
|
| |
Dalton Trans,
(),
889-895.
|
 |
|
|
|
|
 |
R.F.Abdelhamid,
Y.Obara,
Y.Uchida,
T.Kohzuma,
D.M.Dooley,
D.E.Brown,
and
H.Hori
(2007).
Pi-pi interaction between aromatic ring and copper-coordinated His81 imidazole regulates the blue copper active-site structure.
|
| |
J Biol Inorg Chem,
12,
165-173.
|
 |
|
|
|
|
 |
S.Prag,
A.De Arcangelis,
E.Georges-Labouesse,
and
J.C.Adams
(2007).
Regulation of post-translational modifications of muskelin by protein kinase C.
|
| |
Int J Biochem Cell Biol,
39,
366-378.
|
 |
|
|
|
|
 |
S.Ye,
X.Wu,
L.Wei,
D.Tang,
P.Sun,
M.Bartlam,
and
Z.Rao
(2007).
An insight into the mechanism of human cysteine dioxygenase. Key roles of the thioether-bonded tyrosine-cysteine cofactor.
|
| |
J Biol Chem,
282,
3391-3402.
|
 |
|
PDB code:
|
 |
|
|
|
|
|
 |
A.Vanden Wymelenberg,
G.Sabat,
M.Mozuch,
P.J.Kersten,
D.Cullen,
and
R.A.Blanchette
(2006).
Structure, organization, and transcriptional regulation of a family of copper radical oxidase genes in the lignin-degrading basidiomycete Phanerochaete chrysosporium.
|
| |
Appl Environ Microbiol,
72,
4871-4877.
|
 |
|
|
|
|
 |
C.R.Simmons,
Q.Liu,
Q.Huang,
Q.Hao,
T.P.Begley,
P.A.Karplus,
and
M.H.Stipanuk
(2006).
Crystal structure of mammalian cysteine dioxygenase. A novel mononuclear iron center for cysteine thiol oxidation.
|
| |
J Biol Chem,
281,
18723-18733.
|
 |
|
PDB codes:
|
 |
|
|
|
|
|
 |
E.Ficko-Blean,
and
A.B.Boraston
(2006).
The interaction of a carbohydrate-binding module from a Clostridium perfringens N-acetyl-beta-hexosaminidase with its carbohydrate receptor.
|
| |
J Biol Chem,
281,
37748-37757.
|
 |
|
PDB codes:
|
 |
|
|
|
|
|
 |
E.W.Odom,
and
G.R.Vasta
(2006).
Characterization of a binary tandem domain F-type lectin from striped bass (Morone saxatilis).
|
| |
J Biol Chem,
281,
1698-1713.
|
 |
|
|
|
|
 |
E.Zueva,
P.H.Walton,
and
J.E.McGrady
(2006).
Catalytic alcohol oxidation by an unsymmetrical 5-coordinate copper complex: electronic structure and mechanism.
|
| |
Dalton Trans,
(),
159-167.
|
 |
|
|
|
|
 |
F.Michel,
S.Hamman,
F.Thomas,
C.Philouze,
I.Gautier-Luneau,
and
J.L.Pierre
(2006).
Galactose Oxidase models: 19F NMR as a powerful tool to study the solution chemistry of tripodal ligands in the presence of copper(II).
|
| |
Chem Commun (Camb),
(),
4122-4124.
|
 |
|
|
|
|
 |
I.A.Koval,
P.Gamez,
C.Belle,
K.Selmeczi,
and
J.Reedijk
(2006).
Synthetic models of the active site of catechol oxidase: mechanistic studies.
|
| |
Chem Soc Rev,
35,
814-840.
|
 |
|
|
|
|
 |
J.G.McCoy,
L.J.Bailey,
E.Bitto,
C.A.Bingman,
D.J.Aceti,
B.G.Fox,
and
G.N.Phillips
(2006).
Structure and mechanism of mouse cysteine dioxygenase.
|
| |
Proc Natl Acad Sci U S A,
103,
3084-3089.
|
 |
|
PDB code:
|
 |
|
|
|
|
|
 |
L.C.Fortier,
A.Bransi,
and
S.Moineau
(2006).
Genome sequence and global gene expression of Q54, a new phage species linking the 936 and c2 phage species of Lactococcus lactis.
|
| |
J Bacteriol,
188,
6101-6114.
|
 |
|
|
|
|
 |
M.A.Hossain,
F.Thomas,
S.Hamman,
E.Saint-Aman,
D.Boturyn,
P.Dumy,
and
J.L.Pierre
(2006).
Cyclodecapeptides to mimic the radical site of tyrosyl-containing proteins.
|
| |
J Pept Sci,
12,
612-619.
|
 |
|
|
|
|
 |
O.Rotthaus,
F.Thomas,
O.Jarjayes,
C.Philouze,
E.Saint-Aman,
and
J.L.Pierre
(2006).
Valence tautomerism in octahedral and square-planar phenoxyl-nickel(II) complexes: are imino nitrogen atoms good friends?
|
| |
Chemistry,
12,
6953-6962.
|
 |
|
|
|
|
 |
A.Berkessel,
M.Dousset,
S.Bulat,
and
K.Glaubitz
(2005).
Combinatorial approaches to functional models for galactose oxidase.
|
| |
Biol Chem,
386,
1035-1041.
|
 |
|
|
|
|
 |
B.Leuthner,
C.Aichinger,
E.Oehmen,
E.Koopmann,
O.Müller,
P.Müller,
R.Kahmann,
M.Bölker,
and
P.H.Schreier
(2005).
A H2O2-producing glyoxal oxidase is required for filamentous growth and pathogenicity in Ustilago maydis.
|
| |
Mol Genet Genomics,
272,
639-650.
|
 |
|
|
|
|
 |
F.Michel,
S.Torelli,
F.Thomas,
C.Duboc,
C.Philouze,
C.Belle,
S.Hamman,
E.Saint-Aman,
and
J.L.Pierre
(2005).
An unprecedented bridging phenoxyl radical in dicopper(II) complexes: evidence for an s=3/2 spin state.
|
| |
Angew Chem Int Ed Engl,
44,
438-441.
|
 |
|
|
|
|
 |
I.I.Serysheva,
S.L.Hamilton,
W.Chiu,
and
S.J.Ludtke
(2005).
Structure of Ca2+ release channel at 14 A resolution.
|
| |
J Mol Biol,
345,
427-431.
|
 |
|
|
|
|
 |
I.J.Hewitt,
J.K.Tang,
N.T.Madhu,
B.Pilawa,
C.E.Anson,
S.Brooker,
and
A.K.Powell
(2005).
Iron(III) activation hits a [4 + 4] macrocycle.
|
| |
Dalton Trans,
(),
429-432.
|
 |
|
|
|
|
 |
J.Song,
R.C.Tyler,
R.L.Wrobel,
R.O.Frederick,
F.C.Vojtek,
W.B.Jeon,
M.S.Lee,
and
J.L.Markley
(2005).
Solution structure of At3g04780.1-des15, an Arabidopsis thaliana ortholog of the C-terminal domain of human thioredoxin-like protein.
|
| |
Protein Sci,
14,
1059-1063.
|
 |
|
PDB code:
|
 |
|
|
|
|
|
 |
M.D.Swain,
and
D.E.Benson
(2005).
Geometric preferences of crosslinked protein-derived cofactors reveal a high propensity for near-sequence pairs.
|
| |
Proteins,
59,
64-71.
|
 |
|
|
|
|
 |
M.Groll,
M.Bochtler,
H.Brandstetter,
T.Clausen,
and
R.Huber
(2005).
Molecular machines for protein degradation.
|
| |
Chembiochem,
6,
222-256.
|
 |
|
|
|
|
 |
P.Chaudhuri,
K.Wieghardt,
T.Weyhermüller,
T.K.Paine,
S.Mukherjee,
and
C.Mukherjee
(2005).
Biomimetic metal-radical reactivity: aerial oxidation of alcohols, amines, aminophenols and catechols catalyzed by transition metal complexes.
|
| |
Biol Chem,
386,
1023-1033.
|
 |
|
|
|
|
 |
R.A.Ghiladi,
G.M.Knudsen,
K.F.Medzihradszky,
and
P.R.Ortiz de Montellano
(2005).
The Met-Tyr-Trp cross-link in Mycobacterium tuberculosis catalase-peroxidase (KatG): autocatalytic formation and effect on enzyme catalysis and spectroscopic properties.
|
| |
J Biol Chem,
280,
22651-22663.
|
 |
|
|
|
|
 |
R.Schnell,
T.Sandalova,
U.Hellman,
Y.Lindqvist,
and
G.Schneider
(2005).
Siroheme- and [Fe4-S4]-dependent NirA from Mycobacterium tuberculosis is a sulfite reductase with a covalent Cys-Tyr bond in the active site.
|
| |
J Biol Chem,
280,
27319-27328.
|
 |
|
PDB codes:
|
 |
|
|
|
|
|
 |
S.Shleev,
J.Tkac,
A.Christenson,
T.Ruzgas,
A.I.Yaropolov,
J.W.Whittaker,
and
L.Gorton
(2005).
Direct electron transfer between copper-containing proteins and electrodes.
|
| |
Biosens Bioelectron,
20,
2517-2554.
|
 |
|
|
|
|
 |
T.Harashima,
and
J.Heitman
(2005).
Galpha subunit Gpa2 recruits kelch repeat subunits that inhibit receptor-G protein coupling during cAMP-induced dimorphic transitions in Saccharomyces cerevisiae.
|
| |
Mol Biol Cell,
16,
4557-4571.
|
 |
|
|
|
|
 |
G.R.Vasta,
H.Ahmed,
and
E.W.Odom
(2004).
Structural and functional diversity of lectin repertoires in invertebrates, protochordates and ectothermic vertebrates.
|
| |
Curr Opin Struct Biol,
14,
617-630.
|
 |
|
|
|
|
 |
H.Danielsson Thorell,
N.H.Beyer,
N.H.Heegaard,
M.Ohman,
and
T.Nilsson
(2004).
Comparison of native and recombinant chlorite dismutase from Ideonella dechloratans.
|
| |
Eur J Biochem,
271,
3539-3546.
|
 |
|
|
|
|
 |
R.M.Vanacore,
S.Shanmugasundararaj,
D.B.Friedman,
O.Bondar,
B.G.Hudson,
and
M.Sundaramoorthy
(2004).
The alpha1.alpha2 network of collagen IV. Reinforced stabilization of the noncollagenous domain-1 by noncovalent forces and the absence of Met-Lys cross-links.
|
| |
J Biol Chem,
279,
44723-44730.
|
 |
|
PDB codes:
|
 |
|
|
|
|
|
 |
S.E.Deacon,
K.Mahmoud,
R.K.Spooner,
S.J.Firbank,
P.F.Knowles,
S.E.Phillips,
and
M.J.McPherson
(2004).
Enhanced fructose oxidase activity in a galactose oxidase variant.
|
| |
Chembiochem,
5,
972-979.
|
 |
|
PDB code:
|
 |
|
|
|
|
|
 |
X.Li,
D.Zhang,
M.Hannink,
and
L.J.Beamer
(2004).
Crystallization and initial crystallographic analysis of the Kelch domain from human Keap1.
|
| |
Acta Crystallogr D Biol Crystallogr,
60,
2346-2348.
|
 |
|
|
|
|
 |
X.Li,
D.Zhang,
M.Hannink,
and
L.J.Beamer
(2004).
Crystal structure of the Kelch domain of human Keap1.
|
| |
J Biol Chem,
279,
54750-54758.
|
 |
|
PDB code:
|
 |
|
|
|
|
|
 |
C.C.Lee,
A.Kreusch,
D.McMullan,
K.Ng,
and
G.Spraggon
(2003).
Crystal structure of the human neuropilin-1 b1 domain.
|
| |
Structure,
11,
99.
|
 |
|
PDB code:
|
 |
|
|
|
|
|
 |
M.M.Whittaker,
and
J.W.Whittaker
(2003).
Cu(I)-dependent biogenesis of the galactose oxidase redox cofactor.
|
| |
J Biol Chem,
278,
22090-22101.
|
 |
|
|
|
|
 |
M.Wimmerova,
E.Mitchell,
J.F.Sanchez,
C.Gautier,
and
A.Imberty
(2003).
Crystal structure of fungal lectin: six-bladed beta-propeller fold and novel fucose recognition mode for Aleuria aurantia lectin.
|
| |
J Biol Chem,
278,
27059-27067.
|
 |
|
PDB code:
|
 |
|
|
|
|
|
 |
A.M.Hudson,
and
L.Cooley
(2002).
Understanding the function of actin-binding proteins through genetic analysis of Drosophila oogenesis.
|
| |
Annu Rev Genet,
36,
455-488.
|
 |
|
|
|
|
 |
C.M.Hull,
and
J.Heitman
(2002).
Genetics of Cryptococcus neoformans.
|
| |
Annu Rev Genet,
36,
557-615.
|
 |
|
|
|
|
 |
H.Brandstetter,
J.S.Kim,
M.Groll,
P.Göttig,
and
R.Huber
(2002).
Structural basis for the processive protein degradation by tricorn protease.
|
| |
Biol Chem,
383,
1157-1165.
|
 |
|
|
|
|
 |
M.A.Bianchet,
E.W.Odom,
G.R.Vasta,
and
L.M.Amzel
(2002).
A novel fucose recognition fold involved in innate immunity.
|
| |
Nat Struct Biol,
9,
628-634.
|
 |
|
PDB code:
|
 |
|
|
|
|
|
 |
M.Kim,
T.Okajima,
S.Kishishita,
M.Yoshimura,
A.Kawamori,
K.Tanizawa,
and
H.Yamaguchi
(2002).
X-ray snapshots of quinone cofactor biogenesis in bacterial copper amine oxidase.
|
| |
Nat Struct Biol,
9,
591-596.
|
 |
|
PDB codes:
|
 |
|
|
|
|
|
 |
U.Coskun,
G.Grüber,
M.H.Koch,
J.Godovac-Zimmermann,
T.Lemker,
and
V.Müller
(2002).
Cross-talk in the A1-ATPase from Methanosarcina mazei Go1 due to nucleotide binding.
|
| |
J Biol Chem,
277,
17327-17333.
|
 |
|
|
|
|
 |
Z.Jawad,
and
M.Paoli
(2002).
Novel sequences propel familiar folds.
|
| |
Structure,
10,
447-454.
|
 |
|
|
|
|
 |
E.I.Solomon,
P.Chen,
M.Metz,
S.K.Lee,
and
A.E.Palmer
(2001).
Oxygen Binding, Activation, and Reduction to Water by Copper Proteins.
|
| |
Angew Chem Int Ed Engl,
40,
4570-4590.
|
 |
|
|
|
|
 |
H.Brandstetter,
J.S.Kim,
M.Groll,
and
R.Huber
(2001).
Crystal structure of the tricorn protease reveals a protein disassembly line.
|
| |
Nature,
414,
466-470.
|
 |
|
PDB code:
|
 |
|
|
|
|
|
 |
J.P.Klinman
(2001).
How many ways to craft a cofactor?
|
| |
Proc Natl Acad Sci U S A,
98,
14766-14768.
|
 |
|
|
|
|
 |
L.Xie,
and
W.A.van der Donk
(2001).
Homemade cofactors: self-processing in galactose oxidase.
|
| |
Proc Natl Acad Sci U S A,
98,
12863-12865.
|
 |
|
|
|
|
 |
M.A.Halcrow
(2001).
Chemically Modified Amino Acids in Copper Proteins That Bind or Activate Dioxygen The author acknowledges the Royal Society (London) for a University Research Fellowship.
|
| |
Angew Chem Int Ed Engl,
40,
346-349.
|
 |
|
|
|
|
 |
M.A.Sung,
K.Fleming,
H.A.Chen,
and
S.Matthews
(2001).
The solution structure of PapGII from uropathogenic Escherichia coli and its recognition of glycolipid receptors.
|
| |
EMBO Rep,
2,
621-627.
|
 |
|
|
|
|
 |
S.Fukuzumi,
and
S.Itoh
(2001).
Catalytic control of redox reactivities of coenzyme analogs by metal ions.
|
| |
Antioxid Redox Signal,
3,
807-824.
|
 |
|
|
|
|
 |
S.J.Firbank,
M.S.Rogers,
C.M.Wilmot,
D.M.Dooley,
M.A.Halcrow,
P.F.Knowles,
M.J.McPherson,
and
S.E.Phillips
(2001).
Crystal structure of the precursor of galactose oxidase: an unusual self-processing enzyme.
|
| |
Proc Natl Acad Sci U S A,
98,
12932-12937.
|
 |
|
PDB code:
|
 |
|
|
|
|
|
 |
B.Schwartz,
J.E.Dove,
and
J.P.Klinman
(2000).
Kinetic analysis of oxygen utilization during cofactor biogenesis in a copper-containing amine oxidase from yeast.
|
| |
Biochemistry,
39,
3699-3707.
|
 |
|
|
|
|
 |
C.A.Gomez,
L.M.Ptaszek,
A.Villa,
F.Bozzi,
C.Sobacchi,
E.G.Brooks,
L.D.Notarangelo,
E.Spanopoulou,
Z.Q.Pan,
P.Vezzoni,
P.Cortes,
and
S.Santagata
(2000).
Mutations in conserved regions of the predicted RAG2 kelch repeats block initiation of V(D)J recombination and result in primary immunodeficiencies.
|
| |
Mol Cell Biol,
20,
5653-5664.
|
 |
|
|
|
|
 |
D.C.Nelson,
J.Lasswell,
L.E.Rogg,
M.A.Cohen,
and
B.Bartel
(2000).
FKF1, a clock-controlled gene that regulates the transition to flowering in Arabidopsis.
|
| |
Cell,
101,
331-340.
|
 |
|
|
|
|
 |
E.J.Neer,
and
T.F.Smith
(2000).
A groovy new structure.
|
| |
Proc Natl Acad Sci U S A,
97,
960-962.
|
 |
|
|
|
|
 |
M.J.Colaneri,
J.Vitali,
and
J.Peisach
(2000).
Electron spin-echo envelope modulation study of multicrystalline Cu(2+)-insulin: effects of Cd(2+) on the nuclear quadrupole interaction of the Cu(2+)-coordinated imidazole remote nitrogen.
|
| |
Biochemistry,
39,
584-591.
|
 |
|
|
|
|
 |
N.M.Okeley,
and
W.A.van der Donk
(2000).
Novel cofactors via post-translational modifications of enzyme active sites.
|
| |
Chem Biol,
7,
R159-R171.
|
 |
|
|
|
|
 |
T.Kajander,
P.C.Kahn,
S.H.Passila,
D.C.Cohen,
L.Lehtiö,
W.Adolfsen,
J.Warwicker,
U.Schell,
and
A.Goldman
(2000).
Buried charged surface in proteins.
|
| |
Structure,
8,
1203-1214.
|
 |
|
PDB code:
|
 |
|
|
|
|
|
 |
V.Mahadevan,
R.K.Gebbink,
and
T.D.Stack
(2000).
Biomimetic modeling of copper oxidase reactivity.
|
| |
Curr Opin Chem Biol,
4,
228-234.
|
 |
|
|
|
|
 |
A.Durmus,
C.Eicken,
F.Spener,
and
B.Krebs
(1999).
Cloning and comparative protein modeling of two purple acid phosphatase isozymes from sweet potatoes (Ipomoea batatas).
|
| |
Biochim Biophys Acta,
1434,
202-209.
|
 |
|
|
|
|
 |
B.E.Turner,
and
B.P.Branchaud
(1999).
Probing the radical mechanism of galactose oxidase using an ultrafast radical probe.
|
| |
Bioorg Med Chem Lett,
9,
3341-3346.
|
 |
|
|
|
|
 |
C.Eicken,
B.Krebs,
and
J.C.Sacchettini
(1999).
Catechol oxidase - structure and activity.
|
| |
Curr Opin Struct Biol,
9,
677-683.
|
 |
|
|
|
|
 |
D.A.Svistunenko,
A.Rob,
A.Ball,
J.Torres,
M.C.Symons,
M.T.Wilson,
and
C.E.Cooper
(1999).
The electron paramagnetic resonance characterisation of a copper-containing extracellular peroxidase from Thermomonospora fusca BD25.
|
| |
Biochim Biophys Acta,
1434,
74-85.
|
 |
|
|
|
|
 |
H.C.Liang,
M.Dahan,
and
K.D.Karlin
(1999).
Dioxygen-activating bio-inorganic model complexes.
|
| |
Curr Opin Chem Biol,
3,
168-175.
|
 |
|
|
|
|
 |
H.G.Beisel,
S.Kawabata,
S.Iwanaga,
R.Huber,
and
W.Bode
(1999).
Tachylectin-2: crystal structure of a specific GlcNAc/GalNAc-binding lectin involved in the innate immunity host defense of the Japanese horseshoe crab Tachypleus tridentatus.
|
| |
EMBO J,
18,
2313-2322.
|
 |
|
PDB code:
|
 |
|
|
|
|
|
 |
J.L.Johnson,
D.C.Norcross,
P.Arosio,
R.B.Frankel,
and
G.D.Watt
(1999).
Redox reactivity of animal apoferritins and apoheteropolymers assembled from recombinant heavy and light human chain ferritins.
|
| |
Biochemistry,
38,
4089-4096.
|
 |
|
|
|
|
 |
M.A.McGuirl,
and
D.M.Dooley
(1999).
Copper-containing oxidases.
|
| |
Curr Opin Chem Biol,
3,
138-144.
|
 |
|
|
|
|
 |
V.Fülöp,
and
D.T.Jones
(1999).
Beta propellers: structural rigidity and functional diversity.
|
| |
Curr Opin Struct Biol,
9,
715-721.
|
 |
|
|
|
|
 |
X.Huang,
M.P.Cuajungco,
C.S.Atwood,
M.A.Hartshorn,
J.D.Tyndall,
G.R.Hanson,
K.C.Stokes,
M.Leopold,
G.Multhaup,
L.E.Goldstein,
R.C.Scarpa,
A.J.Saunders,
J.Lim,
R.D.Moir,
C.Glabe,
E.F.Bowden,
C.L.Masters,
D.P.Fairlie,
R.E.Tanzi,
and
A.I.Bush
(1999).
Cu(II) potentiation of alzheimer abeta neurotoxicity. Correlation with cell-free hydrogen peroxide production and metal reduction.
|
| |
J Biol Chem,
274,
37111-37116.
|
 |
|
|
|
|
 |
C.Oxvig,
and
T.A.Springer
(1998).
Experimental support for a beta-propeller domain in integrin alpha-subunits and a calcium binding site on its lower surface.
|
| |
Proc Natl Acad Sci U S A,
95,
4870-4875.
|
 |
|
PDB code:
|
 |
|
|
|
|
|
 |
D.P.Molloy,
A.E.Milner,
I.K.Yakub,
G.Chinnadurai,
P.H.Gallimore,
and
R.J.Grand
(1998).
Structural determinants present in the C-terminal binding protein binding site of adenovirus early region 1A proteins.
|
| |
J Biol Chem,
273,
20867-20876.
|
 |
|
|
|
|
 |
M.Karpefors,
P.Adelroth,
A.Aagaard,
H.Sigurdson,
M.Svensson Ek,
and
P.Brzezinski
(1998).
Electron-proton interactions in terminal oxidases.
|
| |
Biochim Biophys Acta,
1365,
159-169.
|
 |
|
|
|
|
 |
M.M.Whittaker,
D.P.Ballou,
and
J.W.Whittaker
(1998).
Kinetic isotope effects as probes of the mechanism of galactose oxidase.
|
| |
Biochemistry,
37,
8426-8436.
|
 |
|
|
|
|
 |
O.Denisenko,
M.Shnyreva,
H.Suzuki,
and
K.Bomsztyk
(1998).
Point mutations in the WD40 domain of Eed block its interaction with Ezh2.
|
| |
Mol Cell Biol,
18,
5634-5642.
|
 |
|
|
|
|
 |
T.A.Kim,
J.Lim,
S.Ota,
S.Raja,
R.Rogers,
B.Rivnay,
H.Avraham,
and
S.Avraham
(1998).
NRP/B, a novel nuclear matrix protein, associates with p110(RB) and is involved in neuronal differentiation.
|
| |
J Cell Biol,
141,
553-566.
|
 |
|
|
|
|
 |
T.Klabunde,
C.Eicken,
J.C.Sacchettini,
and
B.Krebs
(1998).
Crystal structure of a plant catechol oxidase containing a dicopper center.
|
| |
Nat Struct Biol,
5,
1084-1090.
|
 |
|
PDB codes:
|
 |
|
|
|
|
|
 |
T.Wolff,
R.E.O'Neill,
and
P.Palese
(1998).
NS1-Binding protein (NS1-BP): a novel human protein that interacts with the influenza A virus nonstructural NS1 protein is relocalized in the nuclei of infected cells.
|
| |
J Virol,
72,
7170-7180.
|
 |
|
|
|
|
 |
Y.Wang,
J.L.DuBois,
B.Hedman,
K.O.Hodgson,
and
T.D.Stack
(1998).
Catalytic galactose oxidase models: biomimetic Cu(II)-phenoxyl-radical reactivity.
|
| |
Science,
279,
537-540.
|
 |
|
|
|
|
 |
C.Chothia,
T.Hubbard,
S.Brenner,
H.Barns,
and
A.Murzin
(1997).
Protein folds in the all-beta and all-alpha classes.
|
| |
Annu Rev Biophys Biomol Struct,
26,
597-627.
|
 |
|
|
|
|
 |
D.Nurizzo,
M.C.Silvestrini,
M.Mathieu,
F.Cutruzzolà,
D.Bourgeois,
V.Fülöp,
J.Hajdu,
M.Brunori,
M.Tegoni,
and
C.Cambillau
(1997).
N-terminal arm exchange is observed in the 2.15 A crystal structure of oxidized nitrite reductase from Pseudomonas aeruginosa.
|
| |
Structure,
5,
1157-1171.
|
 |
|
PDB code:
|
 |
|
|
|
|
|
 |
J.M.Matthews,
L.D.Ward,
A.Hammacher,
R.S.Norton,
and
R.J.Simpson
(1997).
Roles of histidine 31 and tryptophan 34 in the structure, self-association, and folding of murine interleukin-6.
|
| |
Biochemistry,
36,
6187-6196.
|
 |
|
|
|
|
 |
J.Ng,
R.Li,
K.Morgan,
and
J.Simon
(1997).
Evolutionary conservation and predicted structure of the Drosophila extra sex combs repressor protein.
|
| |
Mol Cell Biol,
17,
6663-6672.
|
 |
|
|
|
|
 |
K.A.Simmen,
A.Newell,
M.Robinson,
J.S.Mills,
G.Canning,
R.Handa,
K.Parkes,
N.Borkakoti,
and
R.Jupp
(1997).
Protein interactions in the herpes simplex virus type 1 VP16-induced complex: VP16 peptide inhibition and mutational analysis of host cell factor requirements.
|
| |
J Virol,
71,
3886-3894.
|
 |
|
|
|
|
 |
T.A.Springer
(1997).
Folding of the N-terminal, ligand-binding region of integrin alpha-subunits into a beta-propeller domain.
|
| |
Proc Natl Acad Sci U S A,
94,
65-72.
|
 |
|
|
|
|
 |
T.F.Smith,
L.Lo Conte,
J.Bienkowska,
C.Gaitatzes,
R.G.Rogers,
and
R.Lathrop
(1997).
Current limitations to protein threading approaches.
|
| |
J Comput Biol,
4,
217-225.
|
 |
|
|
|
|
 |
U.Ermler,
W.Grabarse,
S.Shima,
M.Goubeaud,
and
R.K.Thauer
(1997).
Crystal structure of methyl-coenzyme M reductase: the key enzyme of biological methane formation.
|
| |
Science,
278,
1457-1462.
|
 |
|
PDB code:
|
 |
|
|
|
|
|
 |
C.Ma,
and
B.A.Barry
(1996).
Electron paramagnetic resonance characterization of tyrosine radical, M+, in site-directed mutants of photosystem II(t).
|
| |
Biophys J,
71,
1961-1972.
|
 |
|
|
|
|
 |
D.E.Coleman,
and
S.R.Sprang
(1996).
How G proteins work: a continuing story.
|
| |
Trends Biochem Sci,
21,
41-44.
|
 |
|
|
|
|
 |
F.Mancia,
N.H.Keep,
A.Nakagawa,
P.F.Leadlay,
S.McSweeney,
B.Rasmussen,
P.Bösecke,
O.Diat,
and
P.R.Evans
(1996).
How coenzyme B12 radicals are generated: the crystal structure of methylmalonyl-coenzyme A mutase at 2 A resolution.
|
| |
Structure,
4,
339-350.
|
 |
|
PDB code:
|
 |
|
|
|
|
|
 |
M.E.Anderson,
and
P.A.Lindahl
(1996).
Spectroscopic states of the CO oxidation/CO2 reduction active site of carbon monoxide dehydrogenase and mechanistic implications.
|
| |
Biochemistry,
35,
8371-8380.
|
 |
|
|
|
|
 |
R.M.Wachter,
and
B.P.Branchaud
(1996).
Thiols as mechanistic probes for catalysis by the free radical enzyme galactose oxidase.
|
| |
Biochemistry,
35,
14425-14435.
|
 |
|
|
|
|
 |
G.A.Landrum,
C.A.Ekberg,
and
J.W.Whittaker
(1995).
A ligand field model for MCD spectra of biological cupric complexes.
|
| |
Biophys J,
69,
674-689.
|
 |
|
|
|
|
 |
M.A.Wall,
D.E.Coleman,
E.Lee,
J.A.Iñiguez-Lluhi,
B.A.Posner,
A.G.Gilman,
and
S.R.Sprang
(1995).
The structure of the G protein heterotrimer Gi alpha 1 beta 1 gamma 2.
|
| |
Cell,
83,
1047-1058.
|
 |
|
PDB codes:
|
 |
|
|
|
|
|
 |
M.F.Schmid,
J.Jakana,
P.Matsudaira,
and
W.Chiu
(1995).
Three-dimensional structure of the acrosomal filament of Limulus sperm by 400 kV electron cryomicroscopy.
|
| |
Biophys J,
68,
8S.
|
 |
|
|
|
|
 |
M.Fontecave,
and
H.Eklund
(1995).
Copper amine oxidase: a novel use for a tyrosine.
|
| |
Structure,
3,
1127-1129.
|
 |
|
|
|
|
 |
M.R.Parsons,
M.A.Convery,
C.M.Wilmot,
K.D.Yadav,
V.Blakeley,
A.S.Corner,
S.E.Phillips,
M.J.McPherson,
and
P.F.Knowles
(1995).
Crystal structure of a quinoenzyme: copper amine oxidase of Escherichia coli at 2 A resolution.
|
| |
Structure,
3,
1171-1184.
|
 |
|
PDB code:
|
 |
|
|
|
|
|
 |
R.J.Williams
(1995).
Energised (entatic) states of groups and of secondary structures in proteins and metalloproteins.
|
| |
Eur J Biochem,
234,
363-381.
|
 |
|
|
|
|
 |
S.L.Edwards,
V.L.Davidson,
Y.L.Hyun,
and
P.T.Wingfield
(1995).
Spectroscopic evidence for a common electron transfer pathway for two tryptophan tryptophylquinone enzymes.
|
| |
J Biol Chem,
270,
4293-4298.
|
 |
|
|
|
|
 |
B.A.Barry
(1993).
The role of redox-active amino acids in the photosynthetic water-oxidizing complex.
|
| |
Photochem Photobiol,
57,
179-188.
|
 |
|
|
|
|
 |
C.Chothia,
and
A.G.Murzin
(1993).
New folds for all-beta proteins.
|
| |
Structure,
1,
217-222.
|
 |
|
|
|
|
 |
J.Harder
(1993).
Ribonucleotide reductases and their occurrence in microorganisms: a link to the RNA/DNA transition.
|
| |
FEMS Microbiol Rev,
12,
273-292.
|
 |
|
|
|
|
 |
M.M.Whittaker,
and
J.W.Whittaker
(1993).
Ligand interactions with galactose oxidase: mechanistic insights.
|
| |
Biophys J,
64,
762-772.
|
 |
|
|
|
|
 |
P.J.Kersten,
and
D.Cullen
(1993).
Cloning and characterization of cDNA encoding glyoxal oxidase, a H2O2-producing enzyme from the lignin-degrading basidiomycete Phanerochaete chrysosporium.
|
| |
Proc Natl Acad Sci U S A,
90,
7411-7413.
|
 |
|
|
|
|
 |
R.T.Dean,
S.Gieseg,
and
M.J.Davies
(1993).
Reactive species and their accumulation on radical-damaged proteins.
|
| |
Trends Biochem Sci,
18,
437-441.
|
 |
|
|
|
|
 |
X.Zhang,
J.H.Fuller,
and
W.S.McIntire
(1993).
Cloning, sequencing, expression, and regulation of the structural gene for the copper/topa quinone-containing methylamine oxidase from Arthrobacter strain P1, a gram-positive facultative methylotroph.
|
| |
J Bacteriol,
175,
5617-5627.
|
 |
|
|
|
|
 |
J.A.Tainer,
V.A.Roberts,
and
E.D.Getzoff
(1992).
Protein metal-binding sites.
|
| |
Curr Opin Biotechnol,
3,
378-387.
|
 |
|
|
|
|
 |
J.A.Tainer,
V.A.Roberts,
and
E.D.Getzoff
(1991).
Metal-binding sites in proteins.
|
| |
Curr Opin Biotechnol,
2,
582-591.
|
 |
|
 |
 |
|
The most recent references are shown first.
Citation data come partly from CiteXplore and partly
from an automated harvesting procedure. Note that this is likely to be
only a partial list as not all journals are covered by
either method. However, we are continually building up the citation data
so more and more references will be included with time.
Where a reference describes a PDB structure, the PDB
code is
shown on the right.
|
 |

