 |
PDBsum entry 1e4e

|

|

|
|
 |
Contents |
 |
|
|

|

|
|
|
|
|
|
|
|
|
|
* Residue conservation analysis
|
|
|
|
 |
|
|
 |
 |
 |
 |
Enzyme class:
|
 |
E.C.6.1.2.1
- D-alanine--(R)-lactate ligase.
|
|
 |
 |
 |
 |
 |
Reaction:
|
 |
(R)-lactate + D-alanine + ATP = D-alanyl-(R)-lactate + ADP + phosphate
|
 |
 |
 |
 |
 |
(R)-lactate
Bound ligand (Het Group name = )
matches with 71.43% similarity
|
+
|
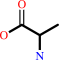 D-alanine
D-alanine
|
+
|
 ATP
ATP
|
=
|
D-alanyl-(R)-lactate
Bound ligand (Het Group name = )
corresponds exactly
|
+
|
 ADP
ADP
|
+
|
 phosphate
phosphate
|
|
 |
 |
 |
 |
 |
 |
 |
 |
 |
 |
|
Molecule diagrams generated from .mol files obtained from the
KEGG ftp site
|
|
 |
 |
 |
 |
 |
 |
 |
 |
 |
 |
 |
 |
 |
 |
 |

|

|

|
| |
|
|
| |
|
DOI no:
|
Proc Natl Acad Sci U S A
97:8921-8925
(2000)
|
|
PubMed id:
|
|
|
|

|
| |
|
The molecular basis of vancomycin resistance in clinically relevant Enterococci: crystal structure of D-alanyl-D-lactate ligase (VanA).
|
|
D.I.Roper,
T.Huyton,
A.Vagin,
G.Dodson.
|
|
|

|
| |
ABSTRACT
|
|

|
| |

|
d-alanine-d-lactate ligase from Enterococcus faecium BM4147 is directly
responsible for the biosynthesis of alternate cell-wall precursors in bacteria,
which are resistant to the glycopeptide antibiotic vancomycin. The crystal
structure has been determined with data extending to 2.5-A resolution. This
structure shows that the active site has unexpected interactions and is distinct
from previous models for d-alanyl-d-lactate ligase mechanistic studies. It
appears that the preference of the enzyme for lactate as a ligand over d-alanine
could be mediated by electrostatic effects and/or a hydrogen-bonding network,
which principally involve His-244. The structure of d-alanyl-d-lactate ligase
provides a revised interpretation of the molecular events that lead to
vancomycin resistance.

|

|
|
|

|
| |
Selected figure(s)
|
|

|
| |
 |
 |

|
 |

|
 |
Figure 2.
Fig. 2. Schematic diagram of the active site of VanA from
the structure showing the interaction of various water molecules
in addition to amino acid side chains. The protein backbone of
the residues in the region of His-244 and Tyr-315 is shown as a
solid black line. Hydrogen bond distances between Tyr-315,
His-244, and the second subsite carboxyl oxygen of the
transition state inhibitor are 2.77 Å and 2.74 Å,
respectively. Two phenylalanine residues (F169 and F294) form
stacking interactions with the adenine nucleotide rings. Several
water molecules in the active site form interactions with the
phosphosphate and magnesium atoms as well as amino acid side
chains. There is an additional hydrogen bond between water 475
and water 371, which is not shown for clarity.
|
 |
Figure 3.
Fig. 3. (a) Comparison of active site waters in E.
facieum VanA, which replace Lys-215 in E. coli DdlB. Magnesium
atoms are shown in gray with water molecules in red. The
structures of VanA and DdlB were superimposed and the relative
positions of waters in VanA and Lys-215 in DdlB compared. Water
475 in VanA takes an equivalent position to the side-chain
nitrogen of Lys-215 in DdlB. Hydrogen-bonding distances between
adjacent water molecules, magnesium atoms, and phosphate atoms
are not shown for clarity. Other water molecules, notably W371,
W569, and W574, coordinate with magnesium ions in the active
site of VanA. (b) A stereo representation and 2 F[o]  F[c] map
showing the active-site residues in contact with the
phosphinophosphinate transition state intermediate. The map is
contoured at 1 F[c] map
showing the active-site residues in contact with the
phosphinophosphinate transition state intermediate. The map is
contoured at 1  by using
the final 2.5-Å resolution map. Residues in the immediate
vicinity of the transition state intermediate are marked. The
two magnesium ions that coordinate with the phosphate ion of the
intermediate and the by using
the final 2.5-Å resolution map. Residues in the immediate
vicinity of the transition state intermediate are marked. The
two magnesium ions that coordinate with the phosphate ion of the
intermediate and the  -phosphate
of ADP are displayed in gray, and water molecules in this
vicinity are displayed in red. Water 475 in VanA takes an
equivalent position to the side-chain nitrogen of Lys-215 in
DdlB. Other water molecules, notably W371 and W574, coordinate
with magnesium ions in the active site of VanA. (c) Stereo
diagram of the hydrogen bonding interacts with and in the
vicinity of the phosphorylated phosphinate inhibitor in the
active site. The Glu-250, Lys-22, Tyr-4315, and His-244
hydrogen-bonding network is shown, making a 2.7-Å hydrogen
bond with the carboxylate oxygen of the inhibitor. This
carboxylate also hydrogen bonds to a conserved serine (316 in
VanA), which is not shown for clarity. The position of His-244
in our structure is such that it cannot make hydrogen-bonding
interactions with the Glu-16 and Ser-177, which are structurally
conserved in comparison to DdlB. The later two amino acids form
important interactions that anchor D-Ala in the first subsite,
an analogous situation to that found in DdlB. -phosphate
of ADP are displayed in gray, and water molecules in this
vicinity are displayed in red. Water 475 in VanA takes an
equivalent position to the side-chain nitrogen of Lys-215 in
DdlB. Other water molecules, notably W371 and W574, coordinate
with magnesium ions in the active site of VanA. (c) Stereo
diagram of the hydrogen bonding interacts with and in the
vicinity of the phosphorylated phosphinate inhibitor in the
active site. The Glu-250, Lys-22, Tyr-4315, and His-244
hydrogen-bonding network is shown, making a 2.7-Å hydrogen
bond with the carboxylate oxygen of the inhibitor. This
carboxylate also hydrogen bonds to a conserved serine (316 in
VanA), which is not shown for clarity. The position of His-244
in our structure is such that it cannot make hydrogen-bonding
interactions with the Glu-16 and Ser-177, which are structurally
conserved in comparison to DdlB. The later two amino acids form
important interactions that anchor D-Ala in the first subsite,
an analogous situation to that found in DdlB.
|
 |
|
|
| |
Figures were
selected
by an automated process.
|
|

|

|
|
 |
 |
|
 |
 |
 |
 |
 |
 |
 |
 |
 |
|
Literature references that cite this PDB file's key reference
|
|
 |
| |
PubMed id
|
 |
Reference
|
 |
|
|
|
 |
Y.Kitamura,
A.Ebihara,
Y.Agari,
A.Shinkai,
K.Hirotsu,
and
S.Kuramitsu
(2009).
Structure of D-alanine-D-alanine ligase from Thermus thermophilus HB8: cumulative conformational change and enzyme-ligand interactions.
|
| |
Acta Crystallogr D Biol Crystallogr,
65,
1098-1106.
|
 |
|
|
|
|
 |
D.Wu,
L.Zhang,
Y.Kong,
J.Du,
S.Chen,
J.Chen,
J.Ding,
H.Jiang,
and
X.Shen
(2008).
Enzymatic characterization and crystal structure analysis of the D-alanine-D-alanine ligase from Helicobacter pylori.
|
| |
Proteins,
72,
1148-1160.
|
 |
|
PDB code:
|
 |
|
|
|
|
|
 |
H.Barreteau,
A.Kovac,
A.Boniface,
M.Sova,
S.Gobec,
and
D.Blanot
(2008).
Cytoplasmic steps of peptidoglycan biosynthesis.
|
| |
FEMS Microbiol Rev,
32,
168-207.
|
 |
|
|
|
|
 |
J.H.Lee,
Y.Na,
H.E.Song,
D.Kim,
B.H.Park,
S.H.Rho,
Y.J.Im,
M.K.Kim,
G.B.Kang,
D.S.Lee,
and
S.H.Eom
(2006).
Crystal structure of the apo form of D-alanine: D-alanine ligase (Ddl) from Thermus caldophilus: a basis for the substrate-induced conformational changes.
|
| |
Proteins,
64,
1078-1082.
|
 |
|
PDB code:
|
 |
|
|
|
|
|
 |
S.Liu,
J.S.Chang,
J.T.Herberg,
M.M.Horng,
P.K.Tomich,
A.H.Lin,
and
K.R.Marotti
(2006).
Allosteric inhibition of Staphylococcus aureus D-alanine:D-alanine ligase revealed by crystallographic studies.
|
| |
Proc Natl Acad Sci U S A,
103,
15178-15183.
|
 |
|
PDB codes:
|
 |
|
|
|
|
|
 |
X.Li,
A.V.Volkov,
K.Szalewicz,
and
P.Coppens
(2006).
Interaction energies between glycopeptide antibiotics and substrates in complexes determined by X-ray crystallography: application of a theoretical databank of aspherical atoms and a symmetry-adapted perturbation theory-based set of interatomic potentials.
|
| |
Acta Crystallogr D Biol Crystallogr,
62,
639-647.
|
 |
|
|
|
|
 |
G.E.Besong,
J.M.Bostock,
W.Stubbings,
I.Chopra,
D.I.Roper,
A.J.Lloyd,
C.W.Fishwick,
and
A.P.Johnson
(2005).
A de novo designed inhibitor of D-Ala-D-Ala ligase from E. coli.
|
| |
Angew Chem Int Ed Engl,
44,
6403-6406.
|
 |
|
|
|
|
 |
E.Potterton,
P.Briggs,
M.Turkenburg,
and
E.Dodson
(2003).
A graphical user interface to the CCP4 program suite.
|
| |
Acta Crystallogr D Biol Crystallogr,
59,
1131-1137.
|
 |
|
|
|
|
 |
B.Henriques Normark,
and
S.Normark
(2002).
Antibiotic tolerance in pneumococci.
|
| |
Clin Microbiol Infect,
8,
613-622.
|
 |
|
|
|
|
 |
J.Pootoolal,
J.Neu,
and
G.D.Wright
(2002).
Glycopeptide antibiotic resistance.
|
| |
Annu Rev Pharmacol Toxicol,
42,
381-408.
|
 |
|
|
|
|
 |
O.H.Ambúr,
P.E.Reynolds,
and
C.A.Arias
(2002).
D-Ala:D-Ala ligase gene flanking the vanC cluster: evidence for presence of three ligase genes in vancomycin-resistant Enterococcus gallinarum BM4174.
|
| |
Antimicrob Agents Chemother,
46,
95.
|
 |
|
|
|
|
 |
N.Woodford
(2001).
Epidemiology of the genetic elements responsible for acquired glycopeptide resistance in enterococci.
|
| |
Microb Drug Resist,
7,
229-236.
|
 |
|
|
|
|
 |
L.M.Dalla Costa,
P.E.Reynolds,
H.A.Souza,
D.C.Souza,
M.F.Palepou,
and
N.Woodford
(2000).
Characterization of a divergent vanD-type resistance element from the first glycopeptide-resistant strain of Enterococcus faecium isolated in Brazil.
|
| |
Antimicrob Agents Chemother,
44,
3444-3446.
|
 |
|
|
|
|
 |
V.L.Healy,
L.S.Mullins,
X.Li,
S.E.Hall,
F.M.Raushel,
and
C.T.Walsh
(2000).
D-Ala-D-X ligases: evaluation of D-alanyl phosphate intermediate by MIX, PIX and rapid quench studies.
|
| |
Chem Biol,
7,
505-514.
|
 |
|
 |
 |
|
The most recent references are shown first.
Citation data come partly from CiteXplore and partly
from an automated harvesting procedure. Note that this is likely to be
only a partial list as not all journals are covered by
either method. However, we are continually building up the citation data
so more and more references will be included with time.
Where a reference describes a PDB structure, the PDB
code is
shown on the right.
|
 |

