 |
PDBsum entry 1bug

|
|
|

|
 |

|

|

|

|

|

|

|

|

|

|

|

|

|

|

|

|

|

|
 |

|

|

|
 |
 |
 |
 |
 |
 |
 |
 |
 |
 |
 |
 |
 |
 |
 |
 |
 |
 |
 |
 |

|

|

|

|

|

|

|

|

|
|
Oxidoreductase
|
PDB id
|
|

|

|
1bug
|

|

|

|

|

|

|

|

|

|

|

|

|

|

|

|

|

|

|

|
|
 |
Contents |
 |
|
|

|

|
|
|
|
|
|
|
|
|
|
* Residue conservation analysis
|
|
|
|
 |
|
|
 |
 |
 |
 |
Enzyme class:
|
 |
E.C.1.10.3.1
- catechol oxidase.
|
|
 |
 |
 |
 |
 |
Reaction:
|
 |
2 catechol + O2 = 2 1,2-benzoquinone + 2 H2O
|
 |
 |
 |
 |
 |
2
×
catechol
Bound ligand (Het Group name = )
matches with 50.00% similarity
|
+
|
 O2
O2
|
=
|
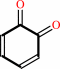 2
×
1,2-benzoquinone
2
×
1,2-benzoquinone
|
+
|
2
×
H2O
|
|
 |
 |
 |
 |
 |
 |
 |
 |
Cofactor:
|
 |
Cu cation
|
 |
 |
 |
 |
 |
 |
 |
|
Molecule diagrams generated from .mol files obtained from the
KEGG ftp site
|
|
 |
 |
 |
 |
 |
 |
 |
 |
 |
 |
 |
 |
 |
 |
 |

|

|

|
| |
|
|
| |
|
DOI no:
|
Nat Struct Biol
5:1084-1090
(1998)
|
|
PubMed id:
|
|
|
|

|
| |
|
Crystal structure of a plant catechol oxidase containing a dicopper center.
|
|
T.Klabunde,
C.Eicken,
J.C.Sacchettini,
B.Krebs.
|
|
|

|
| |
ABSTRACT
|
|

|
| |

|
Catechol oxidases are ubiquitous plant enzymes containing a dinuclear copper
center. In the wound-response mechanism of the plant they catalyze the oxidation
of a broad range of ortho-diphenols to the corresponding o-quinones coupled with
the reduction of oxygen to water. The crystal structures of the enzyme from
sweet potato in the resting dicupric Cu(II)-Cu(II) state, the reduced dicuprous
Cu(I)-Cu(I) form, and in complex with the inhibitor phenylthiourea were
analyzed. The catalytic copper center is accommodated in a central
four-helix-bundle located in a hydrophobic pocket close to the surface. Both
metal binding sites are composed of three histidine ligands. His 109, ligated to
the CuA site, is covalently linked to Cys 92 by an unusual thioether bond. Based
on biochemical, spectroscopic and the presented structural data, a catalytical
mechanism is proposed in which one of the oxygen atoms of the diphenolic
substrate binds to CuB of the oxygenated enzyme.

|

|
|
|

|
| |
Selected figure(s)
|
|

|
| |
 |
 |

|
 |

|
 |
Figure 3.
Figure 3. Active site region of catechol oxidase. a, Stereo
view of the active site region with phenylthiourea bound to the
dicopper center. The sulfur of the inhibitor binds to both
copper ions. In addition the hydrophobic cavity formed by
residues Ile 241, His 244, Phe 261 provides van der Waals
contacts with the aromatic ring of the drug. A stick
presentation of the active site residues of the resting
Cu(II)-Cu(II) state of the enzyme is superimposed in light green
to reveal the conformational change induced by the binding of
PTU. b, Presentation of the molecular surface of the hydrophobic
binding cavity of catechol oxidase showing the two metal ions,
the inhibitor, and Phe 261 in a stick presentation. The
electrostatic surface has been generated omitting these
residues. Areas colored in pink have a negative potential and
areas in purple are of positive potential. c, A close-up of the
hydrophobic binding cavity of catechol oxidase. The images have
been computed using the programs SETOR^30 and SPOCK^31.
|
 |
Figure 4.
Figure 4. Superposition of the dinuclear copper center of sweet
potato catechol oxidase with bound phenylthiourea (PTU) with the
oxygenated form of Limulus polyphemus hemocyanin^19. The side
chains of catechol oxidase are colored by atom type and the
metal-ligating histidine residues of lpHC are shown in green.
The metal-ligating residues forming the CuB binding site are
completely conserved (see also Fig. 6). For the CuA binding site
two amino acid substitutions are found. The HXXXH sequence motif
present in lpHC is changed to HXXXC^92 in catechol oxidase. In
catechol oxidase the side chain of Cys 92 is not coordinated to
CuA and the corresponding free co-ordination site is occupied by
His 109. In hemocyanin phenylalanine Phe 49, located on an  -helix
from the N-terminal domain, blocks the substrate access to the
dicopper center. -helix
from the N-terminal domain, blocks the substrate access to the
dicopper center.
|
 |
|
|

|
| |
The above figures are
reprinted
by permission from Macmillan Publishers Ltd:
Nat Struct Biol
(1998,
5,
1084-1090)
copyright 1998.
|
|
| |
Figures were
selected
by an automated process.
|
|

|

|
|
 |
 |
|
 |
 |
 |
 |
 |
 |
 |
 |
 |
|
Literature references that cite this PDB file's key reference
|
|
 |
| |
PubMed id
|
 |
Reference
|
 |
|
|
|
 |
F.G.Mutti,
M.Gullotti,
L.Casella,
L.Santagostini,
R.Pagliarin,
K.K.Andersson,
M.F.Iozzi,
and
G.Zoppellaro
(2011).
A new chiral, poly-imidazole N8-ligand and the related di- and tri-copper(II) complexes: synthesis, theoretical modelling, spectroscopic properties, and biomimetic stereoselective oxidations.
|
| |
Dalton Trans,
40,
5436-5457.
|
 |
|
|
|
|
 |
J.Liu,
F.Wu,
L.Chen,
J.Hu,
L.Zhao,
C.Chen,
and
L.Peng
(2011).
Evaluation of dihydropyrimidin-(2H)-one analogues and rhodanine derivatives as tyrosinase inhibitors.
|
| |
Bioorg Med Chem Lett,
21,
2376-2379.
|
 |
|
|
|
|
 |
V.L.Davidson
(2011).
Generation of protein-derived redox cofactors by posttranslational modification.
|
| |
Mol Biosyst,
7,
29-37.
|
 |
|
|
|
|
 |
Y.M.Ha,
J.Y.Park,
Y.J.Park,
D.Park,
Y.J.Choi,
J.M.Kim,
E.K.Lee,
Y.K.Han,
J.A.Kim,
J.Y.Lee,
H.R.Moon,
and
H.Y.Chung
(2011).
Synthesis and biological activity of hydroxy substituted phenyl-benzo[d]thiazole analogues for antityrosinase activity in B16 cells.
|
| |
Bioorg Med Chem Lett,
21,
2445-2449.
|
 |
|
|
|
|
 |
Y.Sun,
Z.He,
W.Ma,
and
X.Xia
(2011).
Alternative splicing in the coding region of Ppo-A1 directly influences the polyphenol oxidase activity in common wheat (Triticum aestivum L.).
|
| |
Funct Integr Genomics,
11,
85-93.
|
 |
|
|
|
|
 |
C.Gasparetti,
G.Faccio,
M.Arvas,
J.Buchert,
M.Saloheimo,
and
K.Kruus
(2010).
Discovery of a new tyrosinase-like enzyme family lacking a C-terminally processed domain: production and characterization of an Aspergillus oryzae catechol oxidase.
|
| |
Appl Microbiol Biotechnol,
86,
213-226.
|
 |
|
|
|
|
 |
C.Núñez,
R.Bastida,
A.Macías,
L.Valencia,
N.I.Neuman,
A.C.Rizzi,
C.D.Brondino,
P.J.González,
J.L.Capelo,
and
C.Lodeiro
(2010).
Structural, MALDI-TOF-MS, magnetic and spectroscopic studies of new dinuclear copper(II), cobalt(II) and zinc(II) complexes containing a biomimicking μ-OH bridge.
|
| |
Dalton Trans,
39,
11654-11663.
|
 |
|
|
|
|
 |
H.Choi,
B.H.Han,
Y.S.Shim,
S.K.Kang,
and
C.K.Sung
(2010).
1-(4-Hy-droxy-phen-yl)-3-(3,4,5-tri-methoxy-phen-yl)thio-urea.
|
| |
Acta Crystallogr Sect E Struct Rep Online,
66,
o3303-o3304.
|
 |
|
|
|
|
 |
H.Choi,
Y.S.Shim,
B.H.Han,
S.K.Kang,
and
C.K.Sung
(2010).
1-(2-Hy-droxy-eth-yl)-3-(3-meth-oxy-phen-yl)thio-urea.
|
| |
Acta Crystallogr Sect E Struct Rep Online,
66,
o2487-o2488.
|
 |
|
|
|
|
 |
J.A.Worrall,
and
E.Vijgenboom
(2010).
Copper mining in Streptomyces: enzymes, natural products and development.
|
| |
Nat Prod Rep,
27,
742-756.
|
 |
|
|
|
|
 |
J.L.Muñoz-Muñoz,
F.Garcia-Molina,
R.Varon,
P.A.Garcia-Ruíz,
J.Tudela,
F.Garcia-Cánovas,
and
J.N.Rodríguez-López
(2010).
Suicide inactivation of the diphenolase and monophenolase activities of tyrosinase.
|
| |
IUBMB Life,
62,
539-547.
|
 |
|
|
|
|
 |
M.Fairhead,
and
L.Thöny-Meyer
(2010).
Role of the C-terminal extension in a bacterial tyrosinase.
|
| |
FEBS J,
277,
2083-2095.
|
 |
|
|
|
|
 |
R.K.Das,
B.Saha,
S.M.Rahaman,
and
J.K.Bera
(2010).
Bimetallic catalysis involving dipalladium(I) and diruthenium(I) complexes.
|
| |
Chemistry,
16,
14459-14468.
|
 |
|
|
|
|
 |
B.T.Op't Holt,
M.A.Vance,
L.M.Mirica,
D.E.Heppner,
T.D.Stack,
and
E.I.Solomon
(2009).
Reaction coordinate of a functional model of tyrosinase: spectroscopic and computational characterization.
|
| |
J Am Chem Soc,
131,
6421-6438.
|
 |
|
|
|
|
 |
C.Olivares,
and
F.Solano
(2009).
New insights into the active site structure and catalytic mechanism of tyrosinase and its related proteins.
|
| |
Pigment Cell Melanoma Res,
22,
750-760.
|
 |
|
|
|
|
 |
J.Yoon,
S.Fujii,
and
E.I.Solomon
(2009).
Geometric and electronic structure differences between the type 3 copper sites of the multicopper oxidases and hemocyanin/tyrosinase.
|
| |
Proc Natl Acad Sci U S A,
106,
6585-6590.
|
 |
|
|
|
|
 |
S.R.Kanade,
D.H.Rao,
R.N.Hegde,
and
L.R.Gowda
(2009).
The unique enzymatic function of field bean (Dolichos lablab) D-galactose specific lectin: a polyphenol oxidase.
|
| |
Glycoconj J,
26,
535-545.
|
 |
|
|
|
|
 |
Y.Cong,
Q.Zhang,
D.Woolford,
T.Schweikardt,
H.Khant,
M.Dougherty,
S.J.Ludtke,
W.Chiu,
and
H.Decker
(2009).
Structural mechanism of SDS-induced enzyme activity of scorpion hemocyanin revealed by electron cryomicroscopy.
|
| |
Structure,
17,
749-758.
|
 |
|
PDB codes:
|
 |
|
|
|
|
|
 |
Y.Li,
Y.Wang,
H.Jiang,
and
J.Deng
(2009).
Crystal structure of Manduca sexta prophenoloxidase provides insights into the mechanism of type 3 copper enzymes.
|
| |
Proc Natl Acad Sci U S A,
106,
17002-17006.
|
 |
|
PDB code:
|
 |
|
|
|
|
|
 |
A.Brack,
N.Hellmann,
and
H.Decker
(2008).
Kinetic properties of hexameric tyrosinase from the crustacean Palinurus elephas.
|
| |
Photochem Photobiol,
84,
692-699.
|
 |
|
|
|
|
 |
A.Prokofieva,
A.I.Prikhod'ko,
S.Dechert,
and
F.Meyer
(2008).
Selective benzylic C-C coupling catalyzed by a bioinspired dicopper complex.
|
| |
Chem Commun (Camb),
(),
1005-1007.
|
 |
|
|
|
|
 |
E.J.Land,
C.A.Ramsden,
P.A.Riley,
and
M.R.Stratford
(2008).
Evidence consistent with the requirement of cresolase activity for suicide inactivation of tyrosinase.
|
| |
Tohoku J Exp Med,
216,
231-238.
|
 |
|
|
|
|
 |
E.Jaenicke,
and
H.Decker
(2008).
Kinetic properties of catecholoxidase activity of tarantula hemocyanin.
|
| |
FEBS J,
275,
1518-1528.
|
 |
|
|
|
|
 |
E.Mijangos,
J.Reedijk,
and
L.Gasque
(2008).
Copper(ii) complexes of a polydentate imidazole-based ligand. pH effect on magnetic coupling and catecholase activity.
|
| |
Dalton Trans,
(),
1857-1863.
|
 |
|
|
|
|
 |
F.G.Mutti,
R.Pievo,
M.Sgobba,
M.Gullotti,
and
L.Santagostini
(2008).
Biomimetic modeling of copper complexes: a study of enantioselective catalytic oxidation on d-(+)-catechin and L-( - )-epicatechin with copper complexes.
|
| |
Bioinorg Chem Appl,
(),
762029.
|
 |
|
|
|
|
 |
L.B.Davin,
M.Jourdes,
A.M.Patten,
K.W.Kim,
D.G.Vassão,
and
N.G.Lewis
(2008).
Dissection of lignin macromolecular configuration and assembly: Comparison to related biochemical processes in allyl/propenyl phenol and lignan biosynthesis.
|
| |
Nat Prod Rep,
25,
1015-1090.
|
 |
|
|
|
|
 |
M.Cammarata,
V.Arizza,
C.Cianciolo,
D.Parrinello,
M.Vazzana,
A.Vizzini,
G.Salerno,
and
N.Parrinello
(2008).
The prophenoloxidase system is activated during the tunic inflammatory reaction of Ciona intestinalis.
|
| |
Cell Tissue Res,
333,
481-492.
|
 |
|
|
|
|
 |
Q.Michard,
S.Commo,
J.Rocchetti,
F.El Houari,
A.M.Alleaume,
K.Wakamatsu,
S.Ito,
and
B.A.Bernard
(2008).
TRP-2 expression protects HEK cells from dopamine- and hydroquinone-induced toxicity.
|
| |
Free Radic Biol Med,
45,
1002-1010.
|
 |
|
|
|
|
 |
S.Hirota,
T.Kawahara,
M.Beltramini,
P.Di Muro,
R.S.Magliozzo,
J.Peisach,
L.S.Powers,
N.Tanaka,
S.Nagao,
and
L.Bubacco
(2008).
Molecular basis of the bohr effect in arthropod hemocyanin.
|
| |
J Biol Chem,
283,
31941-31948.
|
 |
|
|
|
|
 |
S.J.Smith,
C.J.Noble,
R.C.Palmer,
G.R.Hanson,
G.Schenk,
L.R.Gahan,
and
M.J.Riley
(2008).
Structural and spectroscopic studies of a model for catechol oxidase.
|
| |
J Biol Inorg Chem,
13,
499-510.
|
 |
|
|
|
|
 |
Y.Tatara,
T.Namba,
Y.Yamagata,
T.Yoshida,
T.Uchida,
and
E.Ichishima
(2008).
Acid activation of protyrosinase from Aspergillus oryzae: homo-tetrameric protyrosinase is converted to active dimers with an essential intersubunit disulfide bond at acidic pH.
|
| |
Pigment Cell Melanoma Res,
21,
89-96.
|
 |
|
|
|
|
 |
A.J.Bortoluzzi,
A.Neves,
and
N.A.Rey
(2007).
2-{[Bis(2-pyridylmethyl)amino]methyl}-6-[(2-hydroxyanilino)methyl]-4-methylphenol: a novel binucleating asymmetric ligand as a precursor to synthetic models for metalloenzymes.
|
| |
Acta Crystallogr C,
63,
o84-o86.
|
 |
|
|
|
|
 |
E.Arias,
J.González,
R.Oria,
and
P.Lopez-Buesa
(2007).
Ascorbic acid and 4-hexylresorcinol effects on pear PPO and PPO catalyzed browning reaction.
|
| |
J Food Sci,
72,
C422-C429.
|
 |
|
|
|
|
 |
H.B.Albada,
F.Soulimani,
B.M.Weckhuysen,
and
R.M.Liskamp
(2007).
Scaffolded amino acids as a close structural mimic of type-3 copper binding sites.
|
| |
Chem Commun (Camb),
(),
4895-4897.
|
 |
|
|
|
|
 |
J.L.Munoz-Munoz,
F.Garcia-Molina,
R.Varon,
J.N.Rodriguez-Lopez,
F.Garcia-Canovas,
and
J.Tudela
(2007).
Kinetic characterization of the oxidation of esculetin by polyphenol oxidase and peroxidase.
|
| |
Biosci Biotechnol Biochem,
71,
390-396.
|
 |
|
|
|
|
 |
K.Born,
P.Comba,
A.Daubinet,
A.Fuchs,
and
H.Wadepohl
(2007).
Catecholase activity of dicopper(II)-bispidine complexes: stabilities and structures of intermediates, kinetics and reaction mechanism.
|
| |
J Biol Inorg Chem,
12,
36-48.
|
 |
|
|
|
|
 |
M.Güell,
and
P.E.Siegbahn
(2007).
Theoretical study of the catalytic mechanism of catechol oxidase.
|
| |
J Biol Inorg Chem,
12,
1251-1264.
|
 |
|
|
|
|
 |
P.Nicholls
(2007).
The oxygenase-peroxidase theory of Bach and Chodat and its modern equivalents: change and permanence in scientific thinking as shown by our understanding of the roles of water, peroxide, and oxygen in the functioning of redox enzymes.
|
| |
Biochemistry (Mosc),
72,
1039-1046.
|
 |
|
|
|
|
 |
S.Bergmann,
J.Markl,
and
B.Lieb
(2007).
The first complete cDNA sequence of the hemocyanin from a bivalve, the protobranch Nucula nucleus.
|
| |
J Mol Evol,
64,
500-510.
|
 |
|
|
|
|
 |
S.R.Kanade,
V.L.Suhas,
N.Chandra,
and
L.R.Gowda
(2007).
Functional interaction of diphenols with polyphenol oxidase. Molecular determinants of substrate/inhibitor specificity.
|
| |
FEBS J,
274,
4177-4187.
|
 |
|
|
|
|
 |
A.C.Rosenzweig,
and
M.H.Sazinsky
(2006).
Structural insights into dioxygen-activating copper enzymes.
|
| |
Curr Opin Struct Biol,
16,
729-735.
|
 |
|
|
|
|
 |
A.Granata,
E.Monzani,
L.Bubacco,
and
L.Casella
(2006).
Mechanistic insight into the activity of tyrosinase from variable-temperature studies in an aqueous/organic solvent.
|
| |
Chemistry,
12,
2504-2514.
|
 |
|
|
|
|
 |
D.Hernández-Romero,
A.Sanchez-Amat,
and
F.Solano
(2006).
A tyrosinase with an abnormally high tyrosine hydroxylase/dopa oxidase ratio.
|
| |
FEBS J,
273,
257-270.
|
 |
|
|
|
|
 |
H.Decker,
T.Schweikardt,
and
F.Tuczek
(2006).
The first crystal structure of tyrosinase: all questions answered?
|
| |
Angew Chem Int Ed Engl,
45,
4546-4550.
|
 |
|
|
|
|
 |
H.Suzuki,
Y.Furusho,
T.Higashi,
Y.Ohnishi,
and
S.Horinouchi
(2006).
A novel o-aminophenol oxidase responsible for formation of the phenoxazinone chromophore of grixazone.
|
| |
J Biol Chem,
281,
824-833.
|
 |
|
|
|
|
 |
I.A.Koval,
K.Selmeczi,
C.Belle,
C.Philouze,
E.Saint-Aman,
I.Gautier-Luneau,
A.M.Schuitema,
M.van Vliet,
P.Gamez,
O.Roubeau,
M.Lüken,
B.Krebs,
M.Lutz,
A.L.Spek,
J.L.Pierre,
and
J.Reedijk
(2006).
Catecholase activity of a copper(II) complex with a macrocyclic ligand: unraveling catalytic mechanisms.
|
| |
Chemistry,
12,
6138-6150.
|
 |
|
|
|
|
 |
I.A.Koval,
P.Gamez,
C.Belle,
K.Selmeczi,
and
J.Reedijk
(2006).
Synthetic models of the active site of catechol oxidase: mechanistic studies.
|
| |
Chem Soc Rev,
35,
814-840.
|
 |
|
|
|
|
 |
I.Bento,
M.A.Carrondo,
and
P.F.Lindley
(2006).
Reduction of dioxygen by enzymes containing copper.
|
| |
J Biol Inorg Chem,
11,
539-547.
|
 |
|
|
|
|
 |
J.P.Piquemal,
and
J.Pilmé
(2006).
Comments on the nature of the bonding in oxygenated dinuclear copper enzyme models.
|
| |
J Mol Struct,
764,
77-86.
|
 |
|
|
|
|
 |
N.Wang,
and
D.N.Hebert
(2006).
Tyrosinase maturation through the mammalian secretory pathway: bringing color to life.
|
| |
Pigment Cell Res,
19,
3.
|
 |
|
|
|
|
 |
S.Bergmann,
B.Lieb,
P.Ruth,
and
J.Markl
(2006).
The hemocyanin from a living fossil, the cephalopod Nautilus pompilius: protein structure, gene organization, and evolution.
|
| |
J Mol Evol,
62,
362-374.
|
 |
|
|
|
|
 |
S.Halaouli,
M.Asther,
J.C.Sigoillot,
M.Hamdi,
and
A.Lomascolo
(2006).
Fungal tyrosinases: new prospects in molecular characteristics, bioengineering and biotechnological applications.
|
| |
J Appl Microbiol,
100,
219-232.
|
 |
|
|
|
|
 |
Y.Matoba,
T.Kumagai,
A.Yamamoto,
H.Yoshitsu,
and
M.Sugiyama
(2006).
Crystallographic evidence that the dinuclear copper center of tyrosinase is flexible during catalysis.
|
| |
J Biol Chem,
281,
8981-8990.
|
 |
|
PDB codes:
|
 |
|
|
|
|
|
 |
A.Jancsó,
Z.Paksi,
N.Jakab,
B.Gyurcsik,
A.Rockenbauer,
and
T.Gajda
(2005).
Solution chemical properties and catecholase-like activity of the copper(II)-Ac-His-His-Gly-His-OH system, a relevant functional model for copper containing oxidases.
|
| |
Dalton Trans,
(),
3187-3194.
|
 |
|
|
|
|
 |
A.M.Hall,
and
S.J.Orlow
(2005).
Degradation of tyrosinase induced by phenylthiourea occurs following Golgi maturation.
|
| |
Pigment Cell Res,
18,
122-129.
|
 |
|
|
|
|
 |
B.Ros,
F.Thümmler,
and
G.Wenzel
(2005).
Comparative analysis of Phytophthora infestans induced gene expression in potato cultivars with different levels of resistance.
|
| |
Plant Biol (Stuttg),
7,
686-693.
|
 |
|
|
|
|
 |
F.Gandía-Herrero,
J.Escribano,
and
F.García-Carmona
(2005).
Characterization of the monophenolase activity of tyrosinase on betaxanthins: the tyramine-betaxanthin/dopamine-betaxanthin pair.
|
| |
Planta,
222,
307-318.
|
 |
|
|
|
|
 |
F.Gandía-Herrero,
M.Jiménez-Atiénzar,
J.Cabanes,
F.García-Carmona,
and
J.Escribano
(2005).
Evidence for a common regulation in the activation of a polyphenol oxidase by trypsin and sodium dodecyl sulfate.
|
| |
Biol Chem,
386,
601-607.
|
 |
|
|
|
|
 |
F.García-Molina,
L.G.Fenoll,
J.C.Morote,
P.A.García-Ruiz,
J.N.Rodríguez-López,
F.García-Cánovas,
and
J.Tudela
(2005).
Opposite effects of peroxidase in the initial stages of tyrosinase-catalysed melanin biosynthesis.
|
| |
Int J Biochem Cell Biol,
37,
1179-1196.
|
 |
|
|
|
|
 |
I.A.Koval,
C.Belle,
K.Selmeczi,
C.Philouze,
E.Saint-Aman,
A.M.Schuitema,
P.Gamez,
J.L.Pierre,
and
J.Reedijk
(2005).
Catecholase activity of a mu-hydroxodicopper(II) macrocyclic complex: structures, intermediates and reaction mechanism.
|
| |
J Biol Inorg Chem,
10,
739-750.
|
 |
|
|
|
|
 |
M.D.Swain,
and
D.E.Benson
(2005).
Geometric preferences of crosslinked protein-derived cofactors reveal a high propensity for near-sequence pairs.
|
| |
Proteins,
59,
64-71.
|
 |
|
|
|
|
 |
M.Merkel,
N.Möller,
M.Piacenza,
S.Grimme,
A.Rompel,
and
B.Krebs
(2005).
Less symmetrical dicopper(II) complexes as catechol oxidase models--an adjacent thioether group increases catecholase activity.
|
| |
Chemistry,
11,
1201-1209.
|
 |
|
|
|
|
 |
N.Gheibi,
A.A.Saboury,
H.Mansuri-Torshizi,
K.Haghbeen,
and
A.A.Moosavi-Movahedi
(2005).
The inhibition effect of some n-alkyl dithiocarbamates on mushroom tyrosinase.
|
| |
J Enzyme Inhib Med Chem,
20,
393-399.
|
 |
|
|
|
|
 |
P.Chaudhuri,
K.Wieghardt,
T.Weyhermüller,
T.K.Paine,
S.Mukherjee,
and
C.Mukherjee
(2005).
Biomimetic metal-radical reactivity: aerial oxidation of alcohols, amines, aminophenols and catechols catalyzed by transition metal complexes.
|
| |
Biol Chem,
386,
1023-1033.
|
 |
|
|
|
|
 |
S.Shleev,
J.Tkac,
A.Christenson,
T.Ruzgas,
A.I.Yaropolov,
J.W.Whittaker,
and
L.Gorton
(2005).
Direct electron transfer between copper-containing proteins and electrodes.
|
| |
Biosens Bioelectron,
20,
2517-2554.
|
 |
|
|
|
|
 |
V.S.Sprakel,
M.C.Feiters,
W.Meyer-Klaucke,
M.Klopstra,
J.Brinksma,
B.L.Feringa,
K.D.Karlin,
and
R.J.Nolte
(2005).
Oxygen binding and activation by the complexes of PY2- and TPA-appended diphenylglycoluril receptors with copper and other metals.
|
| |
Dalton Trans,
(),
3522-3534.
|
 |
|
|
|
|
 |
A.Granata,
E.Monzani,
and
L.Casella
(2004).
Mechanistic insight into the catechol oxidase activity by a biomimetic dinuclear copper complex.
|
| |
J Biol Inorg Chem,
9,
903-913.
|
 |
|
|
|
|
 |
A.W.Tepper,
L.Bubacco,
and
G.W.Canters
(2004).
Stopped-flow fluorescence studies of inhibitor binding to tyrosinase from Streptomyces antibioticus.
|
| |
J Biol Chem,
279,
13425-13434.
|
 |
|
|
|
|
 |
E.Jaenicke,
and
H.Decker
(2004).
Functional changes in the family of type 3 copper proteins during evolution.
|
| |
Chembiochem,
5,
163-169.
|
 |
|
|
|
|
 |
L.G.Fenoll,
M.J.Peñalver,
J.N.Rodríguez-López,
R.Varón,
F.García-Cánovas,
and
J.Tudela
(2004).
Tyrosinase kinetics: discrimination between two models to explain the oxidation mechanism of monophenol and diphenol substrates.
|
| |
Int J Biochem Cell Biol,
36,
235-246.
|
 |
|
|
|
|
 |
T.Uchida,
T.Mogi,
H.Nakamura,
and
T.Kitagawa
(2004).
Role of Tyr-288 at the dioxygen reduction site of cytochrome bo studied by stable isotope labeling and resonance raman spectroscopy.
|
| |
J Biol Chem,
279,
53613-53620.
|
 |
|
|
|
|
 |
K.Shimizu,
S.Yasutake,
and
R.Kondo
(2003).
A new stilbene with tyrosinase inhibitory activity from Chlorophora excelsa.
|
| |
Chem Pharm Bull (Tokyo),
51,
318-319.
|
 |
|
|
|
|
 |
L.Bubacco,
M.Van Gastel,
E.J.Groenen,
E.Vijgenboom,
and
G.W.Canters
(2003).
Spectroscopic characterization of the electronic changes in the active site of Streptomyces antibioticus tyrosinase upon binding of transition state analogue inhibitors.
|
| |
J Biol Chem,
278,
7381-7389.
|
 |
|
|
|
|
 |
M.H.Cho,
S.G.Moinuddin,
G.L.Helms,
S.Hishiyama,
D.Eichinger,
L.B.Davin,
and
N.G.Lewis
(2003).
(+)-Larreatricin hydroxylase, an enantio-specific polyphenol oxidase from the creosote bush (Larrea tridentata).
|
| |
Proc Natl Acad Sci U S A,
100,
10641-10646.
|
 |
|
|
|
|
 |
M.M.Whittaker,
and
J.W.Whittaker
(2003).
Cu(I)-dependent biogenesis of the galactose oxidase redox cofactor.
|
| |
J Biol Chem,
278,
22090-22101.
|
 |
|
|
|
|
 |
T.Naraoka,
H.Uchisawa,
H.Mori,
H.Matsue,
S.Chiba,
and
A.Kimura
(2003).
Purification, characterization and molecular cloning of tyrosinase from the cephalopod mollusk, Illex argentinus.
|
| |
Eur J Biochem,
270,
4026-4038.
|
 |
|
|
|
|
 |
A.E.Todd,
C.A.Orengo,
and
J.M.Thornton
(2002).
Sequence and structural differences between enzyme and nonenzyme homologs.
|
| |
Structure,
10,
1435-1451.
|
 |
|
|
|
|
 |
A.W.Tepper,
L.Bubacco,
and
G.W.Canters
(2002).
Structural basis and mechanism of the inhibition of the type-3 copper protein tyrosinase from Streptomyces antibioticus by halide ions.
|
| |
J Biol Chem,
277,
30436-30444.
|
 |
|
|
|
|
 |
D.López-Serrano,
A.Sanchez-Amat,
and
F.Solano
(2002).
Cloning and molecular characterization of a SDS-activated tyrosinase from Marinomonas mediterranea.
|
| |
Pigment Cell Res,
15,
104-111.
|
 |
|
|
|
|
 |
J.Ackermann,
F.Meyer,
E.Kaifer,
and
H.Pritzkow
(2002).
Tuning the activity of catechol oxidase model complexes by geometric changes of the dicopper core.
|
| |
Chemistry,
8,
247-258.
|
 |
|
|
|
|
 |
J.C.García-Borrón,
and
F.Solano
(2002).
Molecular anatomy of tyrosinase and its related proteins: beyond the histidine-bound metal catalytic center.
|
| |
Pigment Cell Res,
15,
162-173.
|
 |
|
|
|
|
 |
L.G.Fenoll,
J.N.Rodríguez-López,
R.Varón,
P.A.García-Ruiz,
F.García-Cánovas,
and
J.Tudela
(2002).
Kinetic characterisation of the reaction mechanism of mushroom tyrosinase on tyramine/dopamine and L-tyrosine methyl esther/L-dopa methyl esther.
|
| |
Int J Biochem Cell Biol,
34,
1594-1607.
|
 |
|
|
|
|
 |
L.Que,
and
W.B.Tolman
(2002).
Bis(mu-oxo)dimetal "diamond" cores in copper and iron complexes relevant to biocatalysis.
|
| |
Angew Chem Int Ed Engl,
41,
1114-1137.
|
 |
|
|
|
|
 |
T.Nakayama
(2002).
Enzymology of aurone biosynthesis.
|
| |
J Biosci Bioeng,
94,
487-491.
|
 |
|
|
|
|
 |
C.Gerdemann,
C.Eicken,
A.Magrini,
H.E.Meyer,
A.Rompel,
F.Spener,
and
B.Krebs
(2001).
Isozymes of Ipomoea batatas catechol oxidase differ in catalase-like activity.
|
| |
Biochim Biophys Acta,
1548,
94.
|
 |
|
|
|
|
 |
E.I.Solomon,
P.Chen,
M.Metz,
S.K.Lee,
and
A.E.Palmer
(2001).
Oxygen Binding, Activation, and Reduction to Water by Copper Proteins.
|
| |
Angew Chem Int Ed Engl,
40,
4570-4590.
|
 |
|
|
|
|
 |
M.A.Halcrow
(2001).
Chemically Modified Amino Acids in Copper Proteins That Bind or Activate Dioxygen The author acknowledges the Royal Society (London) for a University Research Fellowship.
|
| |
Angew Chem Int Ed Engl,
40,
346-349.
|
 |
|
|
|
|
 |
R.Wegner,
M.Gottschaldt,
H.Görls,
E.G.Jäger,
and
D.Klemm
(2001).
Copper(II) complexes of aminocarbohydrate beta-ketoenaminic ligands: efficient catalysts in catechol oxidation.
|
| |
Chemistry,
7,
2143-2157.
|
 |
|
|
|
|
 |
S.J.Firbank,
M.S.Rogers,
C.M.Wilmot,
D.M.Dooley,
M.A.Halcrow,
P.F.Knowles,
M.J.McPherson,
and
S.E.Phillips
(2001).
Crystal structure of the precursor of galactose oxidase: an unusual self-processing enzyme.
|
| |
Proc Natl Acad Sci U S A,
98,
12932-12937.
|
 |
|
PDB code:
|
 |
|
|
|
|
|
 |
H.Decker,
and
F.Tuczek
(2000).
Tyrosinase/catecholoxidase activity of hemocyanins: structural basis and molecular mechanism.
|
| |
Trends Biochem Sci,
25,
392-397.
|
 |
|
|
|
|
 |
H.Erlandsen,
E.E.Abola,
and
R.C.Stevens
(2000).
Combining structural genomics and enzymology: completing the picture in metabolic pathways and enzyme active sites.
|
| |
Curr Opin Struct Biol,
10,
719-730.
|
 |
|
|
|
|
 |
N.M.Okeley,
and
W.A.van der Donk
(2000).
Novel cofactors via post-translational modifications of enzyme active sites.
|
| |
Chem Biol,
7,
R159-R171.
|
 |
|
|
|
|
 |
T.Nakayama,
K.Yonekura-Sakakibara,
T.Sato,
S.Kikuchi,
Y.Fukui,
M.Fukuchi-Mizutani,
T.Ueda,
M.Nakao,
Y.Tanaka,
T.Kusumi,
and
T.Nishino
(2000).
Aureusidin synthase: a polyphenol oxidase homolog responsible for flower coloration.
|
| |
Science,
290,
1163-1166.
|
 |
|
|
|
|
 |
V.Mahadevan,
R.K.Gebbink,
and
T.D.Stack
(2000).
Biomimetic modeling of copper oxidase reactivity.
|
| |
Curr Opin Chem Biol,
4,
228-234.
|
 |
|
|
|
|
 |
A.Durmus,
C.Eicken,
F.Spener,
and
B.Krebs
(1999).
Cloning and comparative protein modeling of two purple acid phosphatase isozymes from sweet potatoes (Ipomoea batatas).
|
| |
Biochim Biophys Acta,
1434,
202-209.
|
 |
|
|
|
|
 |
C.Eicken,
B.Krebs,
and
J.C.Sacchettini
(1999).
Catechol oxidase - structure and activity.
|
| |
Curr Opin Struct Biol,
9,
677-683.
|
 |
|
|
|
|
 |
M.A.McGuirl,
and
D.M.Dooley
(1999).
Copper-containing oxidases.
|
| |
Curr Opin Chem Biol,
3,
138-144.
|
 |
|
 |
 |
|
The most recent references are shown first.
Citation data come partly from CiteXplore and partly
from an automated harvesting procedure. Note that this is likely to be
only a partial list as not all journals are covered by
either method. However, we are continually building up the citation data
so more and more references will be included with time.
Where a reference describes a PDB structure, the PDB
codes are
shown on the right.
|
 |

